The Manhattan Project
last update: 20 Nov. 2019

General Leslie Groves, J. Robert Oppenheimer, and other scientists examining the site of the Trinity test
Introduction
"The object of the project is to produce a practical military weapon in the form of a bomb...."
On July 13, 2011, U.S. Interior Secretary Kenneth Lee 'Ken' Salazar said “The secret development of the atomic bomb in multiple locations across the United States was an important story and one of the most transformative events in our nation's history”.
He continued, “The Manhattan Project ushered in the atomic age, changed the role of the United States in the world community, and set the stage for the Cold War”.
The eight 'Signature Facilities' that were part of the Manhattan Project were:
The Metallurgical Laboratory ('Met Lab'), University of Chicago with the Chemistry Building and Chicago Pile (CP-1) site
X-10 Graphite Reactor, Oak Ridge, Tennessee
K-25 Gaseous-Diffusion Process Building, Oak Ridge
Y-12 Beta-3 Racetracks, Oak Ridge
B Reactor, Hanford, Washington
Chemical Separations Building (T Plant), Hanford
V-Site Assembly Building, Los Alamos, New Mexico
Trinity Site, Alamogordo, New Mexico.
Within each facility there were, what are called now 'Signature Artefacts', such as the Alpha and Beta calutron magnets and the small calutron in Y-12. Building 9204-3 (Beta 3), still has two Beta calutron racetracks (one still remains in standby today). These instruments or 'tools' are now considered historical artefacts, but what I want to capture in these pages is a little bit about what actually happened between 1939-1945.
As a starting point I have used here, more or less word-for-word, Chapters III and V of “Atomic Energy for Military Purposes” by H. D. Smyth. This report is subtitled “The Official Report on the Development of the Atomic Bomb” 1940-1945. Two very valuable additional reports were “The Manhattan Project” by Terrence R. Fehner and F. G. Gosling (2010), and “Manhattan: The Army and the Atomic Bomb” by Vincent C. Jones (1985). Another valuable and detail report which is publicly available is "A History of the United States Atomic Energy Commission: The New World 1939-1946", by Richard G. Hewlett and Oscar E. Anderson, Jr. I have also started to use a blog called 'Restricted Data' which I find particularly rich and interesting. Additions extracted from other sources are often presented in italics.
Using several different sources inevitably means that there are some overlapping references and some inconsistencies. My original webpage has now been revised and I have tried to extend the descriptions of the research done at the time, in particular in the field of nuclear physics.
The Manhattan Engineering District
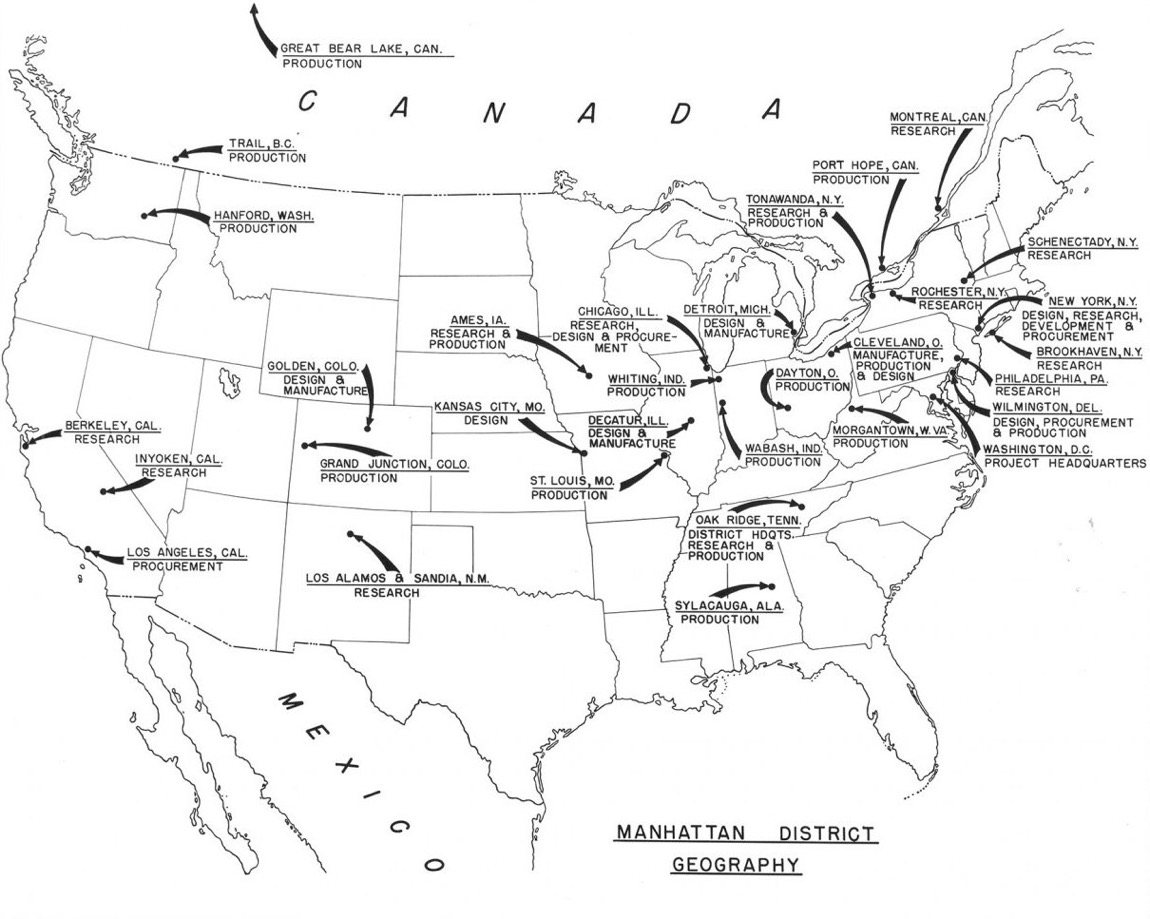
The Manhattan Engineering District (MED, also known as the Manhattan Project) was initially headquartered in New York City. It had no prescribed territorial limits and functioned as a special 'District' (an U.S. Army term) for directing the atomic bomb project. Still today the U.S. Army Corps of Engineers uses 'Division' and 'District', with, for example, Southwestern Division in the U.S., Middle East District, and Europe District.
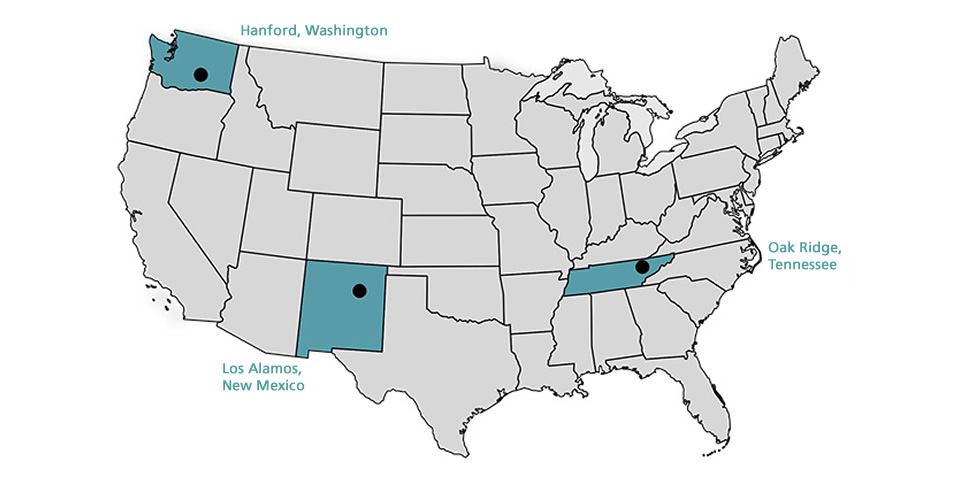
As such, the Manhattan Engineering District supervised research, development and testing, plant construction, and production programs relating to the project. The District also administered numerous laboratories and field installations, including the Clinton Engineer Works at Oak Ridge, Tenn., which performed separation of uranium isotopes; the Hanford Engineer Works at Richland, Wash., which produced plutonium; and the laboratory at Los Alamos, N. Mex., which performed final processing of the fissionable materials and assembled the finished atomic bombs. The District also maintained facilities in more than 30 cities and, during its peak operations, employed more than 129,000 people.
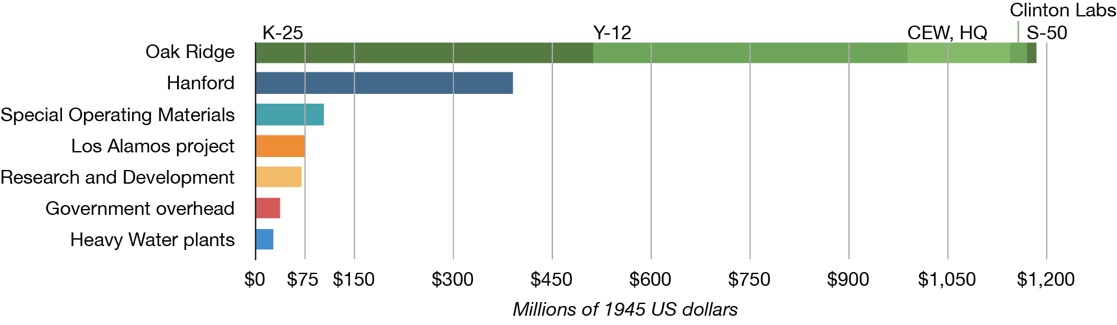
By the end of 1945, the overall cost of the Manhattan Project had reached nearly $2.2 billion, and in 1947 the annual running costs were $300 million. With the creation in 1946 of the AEC Atomic Energy Commission (as part of the Atomic Energy Act) the U.S. Army transferred to the AEC 254 military officers, 1,688 enlisted men, 3,950 government workers, 37,800 contract employees, and 37 installations in 19 different U.S. states and Canada. At the end of the Manhattan Project its size exceeded the U.S. domestic automobile industry. If we just take one site, Oak Ridge, it was employing 12,000 people, and by 1945 it was consuming three times as much electricity as the city of Detroit. According to The Nuclear Security Blog the costs were repartitioned as indicated below, with Oak Ridge taking more than 50%.
According to one estimate, the Manhattan Project cost $2.2 billion from 1942 to 1946 ($22 billion in 2008 dollars), which is much greater than the original cost estimate of approximately $148 million for 1942 to 1944. General Leslie Richard Groves Jr., who managed the Manhattan Project, has written that Members of Congress who inquired about the project were discouraged by the Secretary of War from asking questions or visiting sites. After the project was under way for over a year, in February 1944, War Department officials received essentially a “blank check” for the project from Congressional leadership who “remained completely in the dark” about the Manhattan Project, according to Groves and other experts.
In 2008 dollars, the cumulative cost of the Manhattan project over 5 fiscal years was approximately $22 billion, as compared to approximately $98 billion for the Apollo program over 14 fiscal years. A measure of the U.S. commitment to the programs was their relative shares of the federal outlays during the years of peak funding. For the Manhattan Project, the peak year funding was 1% of federal outlays, and for the Apollo program, 2.2%. Another measure of the commitment was their relative shares of the U.S. gross domestic product (GDP) during the peak years of funding. For both the Manhattan Project and the Apollo program, the peak year funding reached 0.4% of GDP.
General Leslie Richard Groves Jr. (1896-1970), oversaw the construction of the Pentagon and directed the Manhattan Engineering District (1942-1947). He later went on to be vice-president of Sperry Rand.
On July 16, 1945, the first atomic bomb was successfully detonated at Alamogordo, N. Mexico (more usually called the Trinity Site).
On August 6, 1945, and on August 9, 1945, atomic bombs were dropped on Hiroshima and Nagasaki, Japan, respectively.
During the summer of 1944, the problem of postwar control of nuclear energy began to receive serious consideration. In a memorandum of July 27, 1944, James Bryant Conant suggested the creation of a "Commission on Atomic Energy" to be charged with postwar development of nuclear energy for civilian and military purposes. Over the next few months, Conant, along with Vannevar Bush, urged the establishment of such a Commission.
James Bryant Conant (1983-1978), was a chemist, President of Harvard University, and first U.S. Ambassador to West Germany. He worked on poison gases for the U.S. Army in WW I, in particular lewisite. In 1941 he became chairman of the U.S. National Defense Research Committee (NDRC).
Vannevar Bush (1890-1974), was an engineer, and during WW II headed the U.S. Office of Scientific Research and Development. He founded Raytheon, was chairman of Merck, regent of the Smithsonian Institution, and “invented” memex, a proto-hypertext system. He was instrumental in creating the U.S. National Science Foundation.
On May 4, 1945, Henry Lewis Stimson appointed an Interim Committee "to survey and make recommendations on postwar research, development, and controls, as well as legislation necessary to effectuate them". The committee was chaired by the Secretary of War and also included the U.S. Secretary of State James Francis Byrnes, Assistant Secretary of State William Lockhart Clayton, Undersecretary of the Navy Ralph Austin Bard, OSRD's Karl Taylor Compton, Bush, and Conant. The work of the Interim Committee eventually resulted in the establishment of the U.S. Atomic Energy Commission (AEC) by the Atomic Energy Act of August 1, 1946. All phases of nuclear energy research and production came under the control of the AEC on January 1, 1947.
Henry Lewis Stimson (1867-1950) was the U.S. Secretary of War for both WW I and WW II.
On December 31, 1946, Harry S. Truman signed the executive order that transferred peacetime control of the program to the AEC. This marked the end of the Manhattan Project, and transferred ownership of 37 military installations to the civilian commission. The Army also provided the new supervisors of the nation’s nuclear program with a cadre of nearly 3,700 U.S. troops and 4,000 U.S. government civilian employees to provide continuity to the program, just as Groves had called for a year earlier.
At the end of the WW II the Manhattan Engineer District continued for about 6 months, solely as an administrative agency to close out the project and handle personnel assigned to the AEC during the transition period. On December 31, 1946, a joint Army-Navy organisation, the U.S. Armed Forces Special Weapons Project (AFSWP), was established to assume the military functions of the Manhattan Engineering District.
During WW I, Thomas Edison suggested "to the Navy that it should bring into the war effort at least one physicist in case it became necessary to calculate something”. The Manhattan project brought together 1,000’s of physicists, chemists, mathematicians, and engineers. After WW II the effect of science on our society has been incredible, physicists have now become 'experts' on almost everything, and today every government understands that science is a major element in asserting its national power.
How it All Started
The announcement of the hypothesis of fission and its experimental confirmation took place in January 1939. There was immediate interest in the possible military use of the large amounts of energy released in fission. Beginning in 1939, some key scientists expressed concern that Germany might be building an atomic weapon. The early efforts both at restricting publication and at getting government support were stimulated largely by a small group of foreign-born physicists centred on Leo Szilárd and including Eugene Paul Wigner, Edward Teller, Victor Frederick Weisskopf, and Enrico Fermi. At the same time it was proposed that the United States accelerate atomic research in response to the perceived German threat.
The 'Martians', four brilliant scientists who were born in the same neighbourhood in Budapest, Hungary, allegedly earned their nickname from Enrico Fermi. A refugee from Italy himself, Fermi quipped that there must have been a spaceship from Mars that landed in Budapest, dropping off the extraordinarily gifted Edward Teller, Eugene Wigner, John von Neumann, and Leo Szilárd.
One of the remarkable things about the Manhattan Project was that so many “one in a generation” minds gathered at Los Alamos to collaborate on designing and building the bomb. The Manhattan Project boasted eight Nobel Prize laureates: Enrico Fermi, James Chadwick, Niels Henrik David Bohr, Arthur Holly Compton, Ernest Orlando Lawrence, James Franck, Harold Clayton Urey, and Isidor Isaac Rabi. After the war, over a dozen Manhattan Project veterans would go on to win Nobel Prizes, including Hans Albrecht Bethe, Emilio Gino Segré, Eugene Paul Wigner, Richard Phillips Feynman, and Glenn Theodore Seaborg. Then there were brilliant scientists, including Julius Robert Oppenheimer, Chien-Shiung Wu, and John von Neumann, whose valuable contributions were essential to the success of the project and changed science and mathematics forever.
Julius Robert Oppenheimer (1904-1967) was wartime head of Los Alamos, and is often called the “farther of the atomic bomb”. His achievements include the Born–Oppenheimer approximation for molecular wavefunctions, work on the theory of electrons and positrons, the Oppenheimer–Phillips process in nuclear fusion, the Tolman-Oppenhiemer-Volkoff limit for the mass of stars, and the first prediction of quantum tunneling. After WW II, he became director of the Institute for Advanced Study in Princeton.
In the spring of 1939 the group of Szilárd enlisted Niels Bohr's cooperation in an attempt to stop publication of further data by voluntary agreement, but publication continued freely for about another year although a few papers were withheld voluntarily by their authors. At the April 1940 meeting of the Division of Physical Sciences of the U.S. National Research Council, Gregory Breit proposed forming a censorship committee to control publication in all American scientific journals. This arrangement was very successful in preventing publication and was still nominally in effect, in a modified form, through to June 1945.
We have to remember that the 'modern' science community of that time was a web of connections, both as friends and professional. For example, Oppenheimer worked with Bohr, who was teaching Teller. Fellow Hungarian Szilárd met Wigner in 1921 while studying under Einstein, von Laue, and Planck in Berlin. When in January 1939 at the "Fifth Washington Conference on Theoretical Physics: Low Temperature Physics and Superconductivity", Niels Bohr delivered a lecture on splitting the nucleus of uranium, he aroused the concern of numerous conscientious scientists. Leo Szilárd, "obviously concerned, took [Edward Teller] aside", and said, "Let's be careful. Let's not talk about this too much". Teller agreed and "concentrated on returning the conference to the subject of low temperatures". Szilárd, Teller and others were aware that, given the capabilities of German physics and the inclinations of Hitler, the world might be endangered by this scientific discovery.
Strictly speaking Bohr did not deliver a lecture on splitting the nucleus of uranium, what he made was a short unannounced intervention (interruption) at the conference. Attendees were amazed by the announcement. He revealed that Otto Hahn and Friedrich Wilhelm 'Fritz' Strassmann of the Kaiser Wilhelm Institute in Germany, and Lise Meitner with Otto Robert Frisch, both Austrian physicists in exile (Meitner in Sweden and Frisch in Denmark), had been successful in splitting the atom. Among the people sitting in that room were Fermi, Bohr, Urey, Rabi, Teller, Bethe, Roberts, and Johnson, all names we will encounter in these pages on the Manhattan Project.
The first contact with the U.S. government was made by George Brexton Pegram of Columbia University in March 1939. Pegram telephoned to the Navy Department and arranged for a conference between representatives of the Navy and Fermi. The only outcome of this conference was that the Navy expressed interest and asked to be kept informed.
George Brexton Pegram (1876-1958) brokered a meeting between Fermi and the U.S. Navy on the prospects of the atomic bomb. He was chair of the physics department in Columbia University and help found Brookhaven National Laboratory. Fermi, Rabi, Szilárd Anderson, and Zinn all worked in the Columbia physics department.
The next attempt to interest the government was stimulated by Szilárd and Wigner. In July 1939 they conferred with Einstein, and a little later Einstein, Wigner, and Szilárd discussed the problem with Alexander Sachs of New York. In the fall Sachs, supported by a letter from Einstein, explained to President Roosevelt the desirability of encouraging work in this field.
Alexander Sachs (1893-1973) was Lehman Brothers chief economist, and friend of Roosevelt (and some called him also a pompous financier).
On October 11, 1939, Sachs met with the President to discuss the letter written by Albert Einstein the previous August. Einstein had written to inform Roosevelt that recent research on chain reactions utilising uranium made it probable that large amounts of power could be produced by a chain reaction and that, by harnessing this power, the construction of “extremely powerful bombs...” was conceivable. Einstein believed the German government was actively supporting research in this area and urged the United States government to do likewise. Sachs read from a cover letter he had prepared and briefed Roosevelt on the main points contained in Einstein’s letter. Initially the President was noncommittal and expressed concern over locating the necessary funds, but at a second meeting over breakfast the next morning Roosevelt became convinced of the value of exploring atomic energy.
Einstein drafted his famous letter with the help of the Hungarian emigre physicist Leo Szilárd, one of a number of European scientists who had fled to the United States in the 1930’s to escape Nazi and Fascist repression. Szilárd was among the most vocal of those advocating a program to develop bombs based on recent findings in nuclear physics and chemistry. Those like Szilárd and fellow Hungarian refugee physicists Edward Teller and Eugene Wigner regarded it as their responsibility to alert Americans to the possibility that German scientists might win the race to build an atomic bomb and to warn that Hitler would be more than willing to resort to such a weapon.
But Roosevelt, preoccupied with events in Europe, took over two months to meet with Sachs after receiving Einstein’s letter. Szilárd and his colleagues interpreted Roosevelt’s inaction as unwelcome evidence that the President did not take the threat of nuclear warfare seriously.
Roosevelt wrote back to Einstein on October 19, 1939, informing the physicist that he had setup a committee consisting of Sachs and representatives from the Army and Navy to study uranium. Events proved that the President was a man of considerable action once he had chosen a direction. In fact, Roosevelt’s approval of uranium research in October 1939, based on his belief that the United States could not take the risk of allowing Hitler to achieve unilateral possession of “extremely powerful bombs”, was merely the first decision among many that ultimately led to the establishment of the only atomic bomb effort that succeeded in WW II - the Manhattan Project.
The story of Einstein's letter is that Szilárd was convinced that a uranium-graphite experiment might prove successful and it was both imperative to get on with the work and to take steps to keep the uranium ore of the Belgian Congo out of German hands. In talking with his friend Eugene Wigner they thought that Einstein, who knew the Belgian royal family, should write to them. Einstein actually dictated a letter of warning intended for someone of lower rank. But Wigner felt that when contacting a foreign government the U.S. Department of State should be informed. Szilárd felt that it was better still to make some form of direct contact with Washington. Gustav Stolper suggested contacting his friend Alexander Sachs who had ready access to the White House. By the time Szilárd approach Sachs, he had already pointed out to Roosevelt the importance of the work in nuclear physics. It was Sachs who suggested a letter from Einstein to Roosevelt.
Stepping Back in Time
Before looking at how the U.S. responded to Einstein's famous letter, let's look at how the ideas about nuclear fission evolved.
We have to start somewhere, and as good a place as any is in 1930 when Walter Wilhelm Georg Bothe and Herbert Becker bombarded beryllium, boron, fluorine and lithium with alpha-particles from polonium and detected an unusually penetrating radiation (Bothe had already started in 1926 investigating the transmutation of elements by alpha-particles). Bothe and Becker thought this radiation was gamma radiation because unlike alpha particles this radiation was not influenced by an electric field. After their experiments Bothe estimated the photon energy from the degree of absorption of the secondary electrons, but the energy of 'beryllium radiation' varied greatly according to the substance used as absorber.
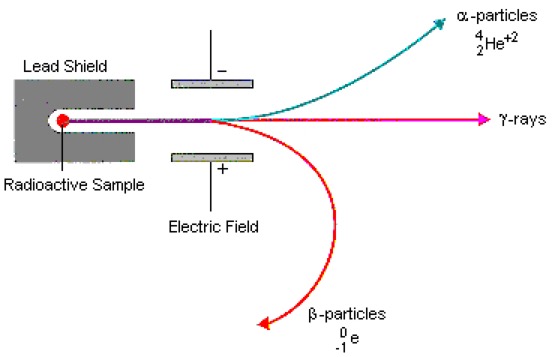
In Paris Irène Joliot-Curie and Frédéric Joliot found that this secondary radiation from beryllium and lithium penetrated through matter even easier than estimated initially by Bothe, and this new radiation passed through a far thicker layer of lead than gamma-rays. Although not inconsistent with gamma radiation, to produce a 5 MeV proton you would need a highly improbable 50 MeV gamma-ray, and at the time Ettore Majorana suggested that a new neutral particle was needed. They continued the study of the ionisation produced by secondary 'beryllium radiation'. Using a ionisation chamber with a thin aluminium detector window they found that the signal increased when they placed hydrogen-containing compounds in front of the detector window. This was due to the ejection of high energy protons, and they continued to assumed that this 'beryllium radiation' was very high energy gamma-rays. They knew that the hypothesis was internally inconsistent, but they assumed that it was a "new mode of interaction of radiation with matter".
Neither Ernest Rutherford not James Chadwick believed in the gamma-ray hypothesis. In fact Chadwick had studied with Johannes Wilhelm 'Hans' Geiger and Bothe at the Physikalisch Technische Reichsanstalt (now PTB) in Berlin. Both Chadwick and Geiger had studied under Rutherford, and Chadwick had been interned in the Ruhleben camp during WW I (Chadwick was helped during his internment by Max Planck, Walther Nernst and Lise Meitner).
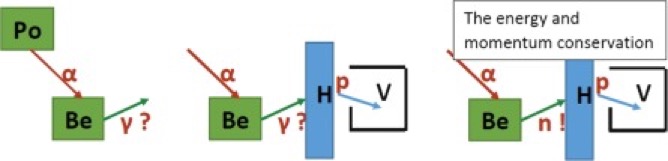
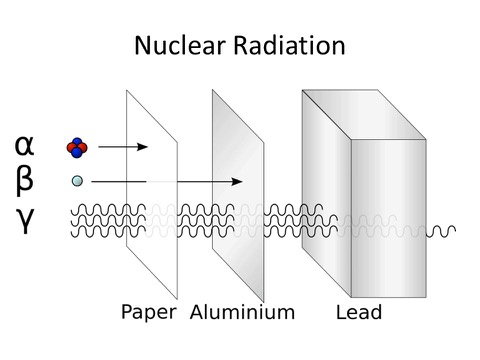
Above we have on the left the Bothe experiment where alpha-particles (in red) from the polonium emit a radiation (in green) which Bothe assumed were gamma-rays. In the middle we have the Joliot-Curie experiment where the hydrogen-based material (H) is hit by radiation (green) and emits high-energy protons. On the right we have Chadwick's experiment where the neutron has now appeared and where energy and momentum are conserved.
We can see the wonderfully simplified presentation of the different experiments, but if we look below at the laboratory of Rutherford we can see that the reality of the time was significantly different.
It was in 1932 that Chadwick bombarded hydrogen and nitrogen with this new 'radiation' and from the recoil energies concluded that the mass of the new particle must be about equal to the that of a proton, but due to its penetrability it must be neutrally charged. He also demonstrated that the gamma-quanta hypothesis was in fact incompatible with energy and momentum conservation. The Joliot-Curies were not immediately convinced, but after additional experiments with a different reaction producing nitrogen they provided additional support for the neutron hypothesis. Chadwick was awarded the 1935 Nobel Prize in Physics for his discovery of the neutron.
Different teams of experimenters had remarked that placing hydrogen-containing materials (e.g. polyethylene) in front of a neutron detector produced a higher signal. The problem is that neutrons are thermalised in hydrogen-containing materials thus increasing the probability of interaction with the detector, but they also eject protons from hydrogen. So in any experiment you could have neutrons, gamma-quanta and protons, and the different type of detectors used by Bothe, the Curies and Chadwick all appeared to be selectively sensitive to different types of radiation. At the time all this made the interpretation more complex. And it must be said that many experimenters did not expect to observe neutrons, on the other hand people such as Chadwick were expecting to see them. Fermi had also noted that his experiments seemed to work better on a wooden table than a marble table, and at the time he suspected that the protons of the wood were slowing the neutrons and so increasing the chance for them to interact with nuclei. In fact his 1938 Nobel Prize for Physics explicitly noted "related discovery of nuclear reactions brought about by slow neutrons".
The appearance of the neutron revolutionised the 'classical' proton-electron model of the atom, and quickly both Dimitri Ivanenko and Werner Heisenberg proposed new neutron-proton models of the atom. Heisenberg saw the neutron as a proton-electron composite, but he introduced the idea of nuclear exchange forces that bind nucleons and the idea that protons and neutrons were different quantum states of the same particle. Ivanenko proposed that atomic nuclei consisted only of protons and neutrons and he was also the first to propose the nuclear shell model, which later led to the postulate of a strong force to overcome electro-magnetic repulsion and bind the protons inside the atomic nucleus. Already in 1930 it was George Gamow that had proposed a semi-empirical liquid-drop model for the atomic nucleus. And it was in 1935 that the Bethe-Weiszsäcker formula was used to calculate the properties (including mass) of an atomic nucleus from its number of protons and neutrons.
From 1928 Irène Joliot-Curie and her husband Frédéric had studied atomic nuclei, but had failed to identify both the positron and the neutron (two missed opportunities for Nobel Prizes). However in 1934 they irradiated several light elements, including a natural stable isotope of aluminium (aluminium-27), with alpha particles from a polonium source and produced unstable isotopes of phosphorus (phosphorus-30). This opened the door to the quick and cheap production of radioisotopes (and third-time lucky for the 1935 Nobel Prize for Chemistry). And if you are attentive you will also have noticed that the creation of the 'radio-phosphorus' atom is accompanied by the emission of a neutron. Phosphorus-30 is unstable and in decaying to stable silicon-30 is a positron emitter with a 2.5 minute half-life. At the time Irène suggested that different nuclear reactions would produce other elements with other bombarding particles (protons, deuterons, and neutrons) but she also affirmed that nuclear fission was impossible.
When Enrico Fermi saw the 1934 results of Irène and Frédéric Joliot-Curie he immediately decided to switch to experimental neutron physics. He understood immediately that the neutron is unaffected by the Coulomb barrier so it could be used to induce nuclear reactions at lower energies and with all types of targets. This idea was key in that Fermi also saw the neutron not just as a product of a reaction, but also as an agent of a reaction.
These two lines are a kind of 'official' description, but the reality was somewhat different. The Physics Institute at the University of Rome 'La Sapienza' had produced some interesting theoretical work but had been unable to build a coherent body of experimental work. In 1932 already Fermi was debating whether to continue to 'fool around' with the Wilson cloud chamber, or to become (again) a theoretician, or to continue in the field of spectroscopy which was gradually being 'worked out'. Finally Fermi with Franco Rasetti (two of the so-called 'Via Panisperna' boys, a name taken from the street where the laboratory was situated in Rome) decided on a research program in nuclear physics. Facilities were so poor in the laboratory that equipment building was 'farmed out', but they did manage to construct a large cloud chamber that worked perfectly. Their research budget at the time was about $2,000 to $3,000 per year, as compared to $200-500 in other Italian universities. However through 1933 the group continued to published important theoretical work, e.g. on nuclear spin, on neutron-proton interaction, on the theory of nuclei, and Fermi published his famous beta-decay theory (published in Italy and Germany, but refused by Nature as being "too speculative to be of any interest to the reader").
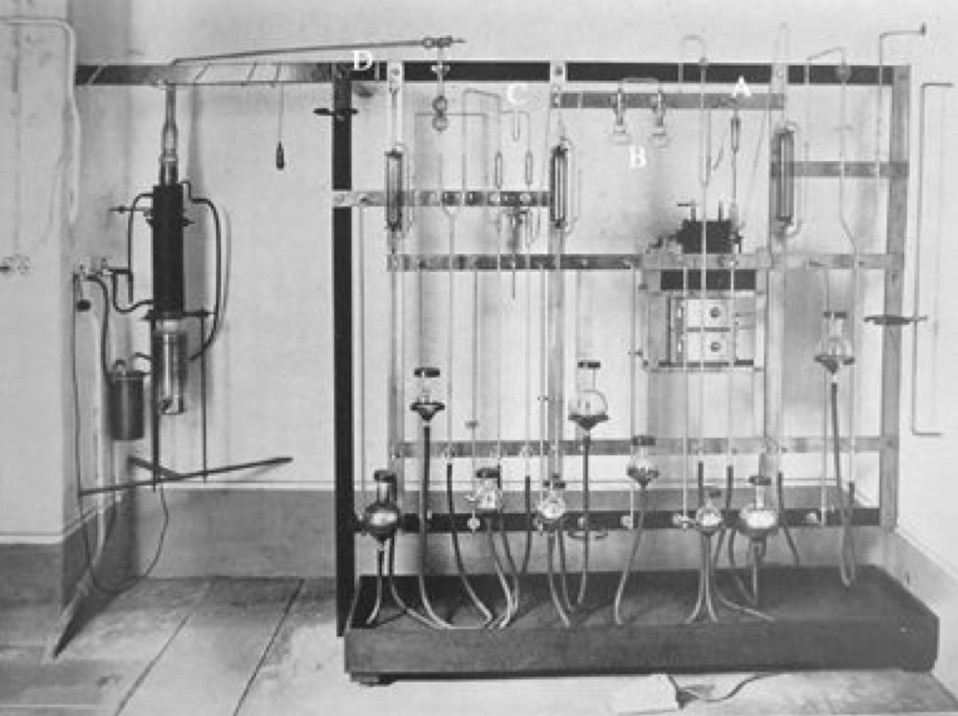
Often overlooked, a group that planned to do work in the nuclear field needed radioactive sources and detectors. This was not something that could be improvised. Obtaining radioactive substances was not an easy task. In 1933 Italian universities only had a few milligrams of radium salts, totally insufficient for a nuclear research program. On the other hand the neighbouring Institute of Public Health held more than 1.6 grams of radium salts for medical therapies (and 300 milligrams in reserve). The Physics Institute managed to get their hands on these radium salts and first extracted some radon (see the apparatus above) and more importantly made some polonium sources. As a pure alpha emitter polonium was the preferred research source at the time. As luck would have it to extract polonium the radium salts must not be fresh, and the salts held with the Health Institute were at least 14 years old. After learning the techniques in Kaiser Wilhelm Institut in Berlin and at Marie Curie's laboratories in Paris the team were able to extract about 110 mCi which at the time made it one of the worlds strongest radiation sources. With these sources Rome could start real experimental work in late 1933.
In parallel with the work on the sources, the team needed to acquire expertise in radiation detector systems. For this members of the team spent time in Florence and with Walther Bothe in Berlin. Below we have a typical ionisation chamber from the period, with the hollow cylinder for the specimen.

We can see now that when the discovery of artificial radioactivity was published in 1934, Fermi and his team in Rome were ready.
With Fermi it is always difficult to follow his work since when he was in Rome many of his interests overlapped and were intertwined. We have to think that during the early 1930's experimental physics was focussed on looking at reactions producing particles of specific predictable energies. Theoreticians were not able to explain why a continuous spectrum of electrons were emitted during beta-decay. If beta-decay of a nucleus generated a single particle then energy conservation implied that the electrons must have a well-defined energy. But the observed energy spectrum was continuous, so did that mean that energy was not conserved?
Wolfgang Ernst Pauli had already suggested that another particle might be emitted simultaneously, but of course no one had seen this experimentally. But they would not see it if it were of very high energy, i.e. going through the detector with very high penetrating power and losing no energy that could be converted into a signal. Pauli actually joked about the particle, since its did not leave a signal he would not write about it. In 1933 Francis Perrin analysed the data on beta-decay and suggested a new particle (now called the neutrino) of almost zero mass and travelling at close the speed of light. Fermi quickly followed with his theory of beta-decay in which a neutron is transformed into a proton, an electron and an antineutrino. Essentially Fermi had invented the weak interaction.
So within a few years two new fundamental interactions (strong and weak) were added to gravity and electro-magnetism.
So now we have Fermi wanting to repeat the experiments of Joliot-Curie but with neutrons. Furthermore Fermi specifically thought that because of the general instability of heavy nuclei such as thorium and uranium they might see successive transformations.

His source was a beryllium powder mixed with radon in a small glass bulb (see above). The alpha particles from the radon hit the beryllium, and produce neutrons. A few months later he reported on the discovery of neutron-induced radioactivity on 22 different elements (a technique now known as neutron activation analysis). The Joliet-Curies went on to confirmed Fermi's results for silver, silicon, zinc, iodine, and iron. From there 'everyone' started to discover other artificial transmutations so rapidly that within months there was a whole family of artificially produced radio-elements.
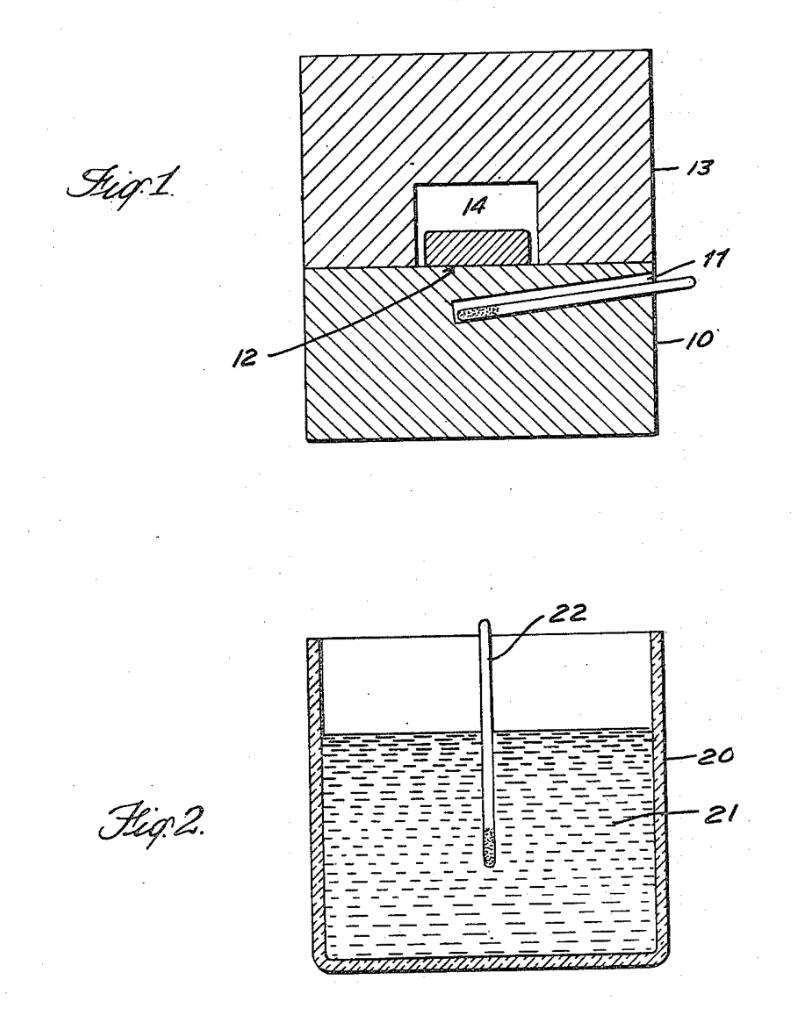
Above we can see Fermi's original patent for the 'production of radioactive substances' where in Fig. 1 there is a cylindrical paraffin block (10) proved with a hole (11) in which a neutron source is inserted (this would be the radon and beryllium bulb). The material being irritated (12) is placed above the source on the paraffin block and is covered by a second paraffin block (13) having a central opening (14). As an example this would be a block about 24 cm in diameter, about 14 cm in height, with the neutron source about 2 cm under the upper surface. Fig. 2 is an alternative where the substance being irradiated is dissolved or dispersed in the energy reducing or dispersing material (21), in a container (20) with a neutron source placed in the middle (22).
Fermi also bombarded thorium and uranium with neutrons and (incorrectly) concluded that he had created two new elements, hesperium and audonien (see his notes below). Fermi had looked at a particular 13-minute decay period produced by neutron irradiation of uranium. Radiochemical tests had excluded that this radiation came from isotopes with a Z between 86 and 92, and therefore it appeared totally logical that the radiation must come from the element 93 or 94. At the time it was thought that fission was theoretically impossible, but they did expect elements with higher atomic numbers to be formed by neutron bombardment of lighter elements.
Rutherford wrote to Fermi on the April 23, 1934 congratulating him on his "successful escape from theoretical physics".
Following Fermi's publication there were a number of criticism as well as some erroneous confirmations. However the harshest criticism came from the chemist Ida Noddack who worked with her husband in the Physikalisch Technische Reichsanstalt in Berlin (they had previously discovered the element rhenium). Her argument was that it was insufficient to eliminate neighbouring elements of uranium and she suggested that the decaying nuclei might be "heavy fragments which are isotopes of known elements". So the idea of nuclear fission was born on September 10, 1934, despite the fact that everyone thought the idea impossible. She was not able to provide any experimental data or theoretical model supporting her criticism, and did nothing to prove her hypothesis. Meitner and Frisch actually published a paper stating that the idea of fission fragments (not in those words) was rejected for "physical reasons" but the chemical evidence "was not entirely clear-cut".
The work of Fermi was followed and reproduced by both the team in Berlin and in Paris. Irradiating uranium and thorium with both slow and fast neutron produces a multitude of nuclei/isotopes/isomers across a series of elements with similar chemical properties. But as the data came in so the internal contradictions became more evident. The problem became more evident, but not the solution. Meitner, Hahn and Strassmann did have doubts and they finally decided to disentangle all the new neutron-induced radioactivities. Physics up to WW II was a wonderfully open and international domain. Hahn had worked with William Ramsay in England in 1904, and it was Ramsey that convinced Hahn to take up radiochemistry and found him his first posting at the University of Berlin. But before moving to Berlin Hahn spent a few months with Rutherford in Montreal, then upon arriving in Berlin he met Lise Meitner in 1906 who had just arriving from working with Ludwig Boltzmann in Vienna. They joined forces to study radioactivity, and formed one of the most productive scientific partnership in the 20th C.
Lise Meitner was the second woman to obtain a degree in physics in Austria, and she was the first woman to 'habilitieren' in physics at the University of Berlin. Her first lecture in 1922 was enticed "On the importance of radioactivity on cosmic processes", which the Berlin newspapers announced as a contribution on cosmetic physics. From September 1933, as a Jew, Meitner lost her teaching post and when Austria was annexed in March 1938 she automatically 'became' a German citizen. Fortunately Meitner left Berlin on July 14, 1938, otherwise she might not have survived the 'Crystal Night' (November 9-10, 1938). Between 1938 and 1945 the Jewish population of Berlin fell from 170,000 to 5,000.
Over the years there had been considerable competition between Irène Joliot-Curie and Paul Savitch in Paris and the team Hahn, Meitner and Strassmann in Berlin. However they were all working on the idea that neutron irradiation of uranium would produce several 'transuranium' elements and a number of other products (still to be properly identified) through successive alpha decays. On November 8, 1938 Hahn and Strassmann published a paper claiming that the neutron irradiation of uranium would produce thorium-235 and radium-231 by successive alpha-decays. Bohr, Frisch and Meitner (in Stockholm at that time) told the authors that what they were proposing was unlikely purely on energetic considerations. So Hahn and Strassmann went back to the drawing board and decided to test what they thought was thorium and radium (and actinium as a transitory isotope), only to find that the three 'fragments' were in fact barium, lanthanum, and caesium. You have to imagine what this meant. How could a little thing like a neutron break in parts a heavy nucleus? And the worlds scientists were discussing the exact opposite process, the creation of heavier transuranium elements. The 1939 paper of Hahn and Strassmann clearly announced that nuclear fission had been discovered, but whilst the 'solution' was chemical, now physics would have to explain how a nucleus could split into two heavy fragments of about equal size.
On December 19, 1938 Meitner was the first to learn of the new results of Hahn and Strassmann, and Hahn asked her if she could explain what they had found (he actually asked for a 'fantastic explanation'). Meitner was in Kungälv (north of Goteborg) spending New Year with her nephew Otto Robert Frisch. On January 16, 1939 Meitner and Frisch submitted a paper to Nature describing what the physical process might be. The idea was that the nucleus acts like a liquid drop, and with a violent impact could divide into two smaller drops. They even calculated the kinetic energy release to be about 200 MeV.
Frisch immediately realised that this release of energy must be easily measurable in an ionisation chamber. He returned to Copenhagen (where he worked with Bohr) and performed the experiment demonstrating that the kinetic energies were about 71 MeV per fragment. Thus nuclear fission was also physically confirmed.
Frisch told Bohr about the discovery and gave a first draft of their explanation of nuclear fission. Bohr left for America on January 7, 1939, travelling with Léon Rosenfeld (on the liner SS Drottningholm). On the January 16, 1939 Rosenfeld talked about the results at Princeton's Journal club, and Bohr gave the news to a larger group of physicists at the Fifth Washington Conference on Theoretical Physics (January 26, 1939). The full story was published by the Physical Review on February 15, 1939.
By June 26, 1939 Niels Bohr and John Archibald Wheeler had published the basic theory of nuclear fission. In fact by the end of 1939 nearly 100 scientific articles had been published on the phenomena of fission.
An excess of the number of neutrons over the number of protons in a nucleus increases as a function of atomic weight, so it appeared natural that really heavy nuclei like uranium could emit not one but a few neutrons. This would mean that one neutron 'in' could produce several neutrons 'out'. This was a multiplying process, or as they said fission can run 'in chain'. Multiple teams found that neutrons were liberated in the nuclear fission of uranium induced by slow neutron bombardment, and that the secondary neutrons had directions, energies, and temporal properties that were not directly related to the primary neutron process.
Already in his 1935 Nobel Prize lecture Frédéric Joliot-Curie had discussed the idea of a nuclear chain reaction and the important release of energy. In 1939 he and Hans von Halban, Lew Kowarski and Francis Perrin placed a source of neutrons in the centre of a copper sphere immersed in water. They filled the sphere with uranium oxide, or water, or a mixture, and measured the neutron fluxes in the various cases. This was to all intents and purposes a so-called sub-critical assembly. They were able to measure the mean number of neutrons produced per fission as 3.5+/-0.7 (today's exact value is 2.41). This multiplicity established the possibility of nuclear chain reactions and thus nuclear energy production. The team even noted that had they made the sphere bigger fewer neutrons would have escaped. And the neutron multiplication factor would have increased until it exploded. Francis Perrin estimated this as the critical mass without a reflector around it.
These experiments used an external neutron flux to provide the initial fission events, but the model of Bohr and Wheeler predicted that a gamma-quanta could also initiate a chain reaction, and this was observed in 1940. At the same time Georgy Flyorov and Konstantin Petrzhak in Leningrad announced that a heavy enough nucleus could fission spontaneously, without an initial kick. This meant that a fission chain reaction could occur by itself once the conditions of critical mass were met.
Thought experiments abounded. You would need a low absorbing neutron moderator and reflector, like graphite or heavy-water. A neutron absorber (or 'poison'), such as cadmium, could allow some kind of control so as to maintain a steady chain reaction. You could produce radioisotopes. You could produce energy. In May 1939 Joliot, Halban and Kowarski actually filed patents for power production and nuclear explosions. At the same time, from July 1938, there was an increasing exodus of Jewish scientists from mainland Europe to the United Kingdom and even more so to the U.S. By 1940 the whole topic of nuclear physics had become highly confidential.
Now back to the Manhattan Project.
The Uranium Committee
The U.S. President appointed a committee, known as the 'Advisory Committee on Uranium', consisting of Lyman James Briggs (director of the U.S. National Bureau of Standards) as chairman, Colonel K. F. Adamson of the Army Ordnance Department, and Commander G. C. Hoover of the Navy Bureau of Ordnance, and requested this committee to look into the problem. From June 1940 there was a technical sub-committee with Harold Clayton Urey (1934 Nobel Prize for Chemistry), Ross Gunn, George Pergram, Merle Anthony Tuve, Jesse Wakefield Beams, and Gregory Breit. The committee on uranium was the only committee that had official status up to the time of the organisation of the National Defence Research Committee in June 1940. The committee met very informally and included various additional scientific representatives in its meetings.
The first meeting of the Uranium Committee was on October 21, 1939 and included, besides the committee members, F. L. Mohler, Sachs, Szilárd, Wigner, Teller, and Richard Brooke Roberts. The result of this meeting was a report dated November 1, 1939, and transmitted to President Roosevelt by Briggs, Adamson, and Hoover. This report made eight recommendations, and specifically mentioned both atomic power and an atomic bomb as possibilities. It recommended procurement of 4 tons of graphite and 50 tons of uranium oxide for measurements of the absorption cross-section of carbon.
The first transfer of funds ($6,000) from the Army and Navy to purchase materials in accordance with the recommendation of November 1, 1939, is reported in a memorandum from Briggs to General Edwin Martin Watson (President Roosevelt's aide) on February 20, 1940. The next meeting of the 'Advisory Committee on Uranium' was on April 28, 1940 and was attended by Sachs, Wigner, Pegram, Fermi, Szilárd, Briggs, Admiral Harold Gardiner Bowen, Colonel Adamson, and Commander Hoover.
The Army and Navy each gave $3,000 to National Bureau of Standards, which gave the money to Columbia University (i.e. Fermi), who used it to buy an 'inordinate' quantity of graphite. The official line was that the Army was only convinced to allocate these fund when it learned that the President “was interested in this project”. However the story is a bit different. It was Teller who had referred incidentally to the amount of money that could be spent profitably in the months ahead. Colonel Adamson noted that usually it took two wars to develop a new weapon, and it was morale that won wars, not arms. Wigner replied that if armaments were so unimportant a cut of 30% in the defence budget might be a good idea. Apparently Adamson snapped that "you'll get your money".
$6,000 today does not sound like very much, but at the time (February 1940) it reflected the importance attached to the Fermi-Szilárd pile experiments already underway at Columbia University. Building upon the work performed in 1934 demonstrating the value of moderators in producing slow neutrons, Fermi thought that a mixture of the right moderator and natural uranium could produce a self-sustaining chain reaction. Fermi and Szilárd increasingly focused their attention on carbon in the form of graphite. Perhaps graphite could slow down, or moderate, the neutrons coming from the fission reaction, increasing the probability of their causing additional fissions in sustaining the chain reaction. A pile containing a large amount of natural uranium could then produce enough secondary neutrons to keep a reaction going.
There was, however, a large theoretical gap between building a self-generating pile and building a bomb. Although the pile envisioned by Fermi and Szilárd could produce large amounts of power and might have military applications (powering naval vessels, for instance), it would be too big for a bomb. It would take separation of uranium-235 or substantial enrichment of natural uranium with uranium-235 to create a fast-neutron reaction on a small enough scale to build a usable bomb. While certain of the chances of success in his graphite power pile, Fermi, in 1939, thought that there was “little likelihood of an atomic bomb, little proof that we were not pursuing a chimera".
By the time of this meeting two important new factors had come to light. First, it had been discovered that the uranium fission caused by neutrons of thermal velocities occurred in the uranium-235 isotope only. Second, it had been reported that a large section of the Kaiser Wilhelm Institute in Berlin had been set aside for research on uranium.
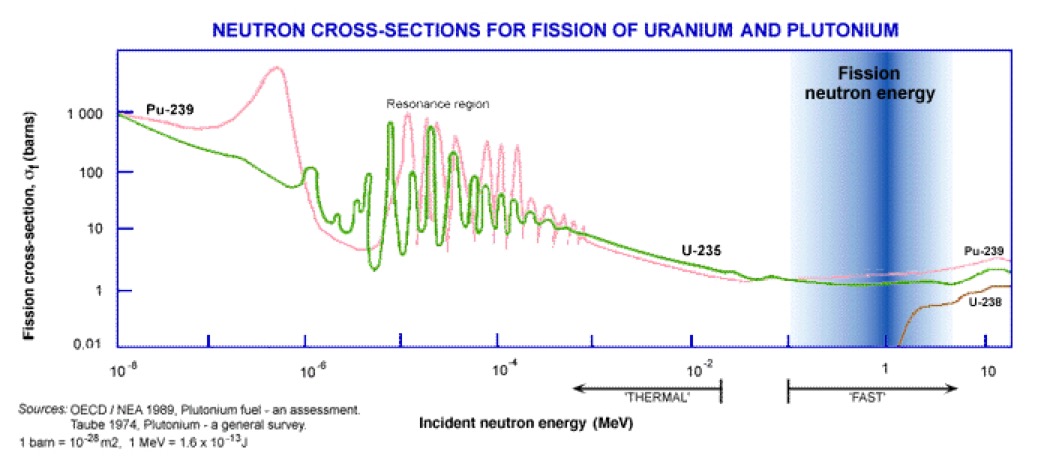
Fission may take place in any of the heavy nuclei after capture of a neutron. However, low-energy (slow, or thermal) neutrons are able to cause fission only in those isotopes of uranium and plutonium whose nuclei contain odd numbers of neutrons (e.g. U-233, U-235, and Pu-239). The fission cross-section increases dramatically (when competing with the capture cross-section) for thermal neutron (<0.025 eV) on U-235 than for higher energies (1/v dependence).
Within the next few weeks a number of people, particularly Sachs, urged the importance of greater support and of better organisation. Their hand was strengthened by the Columbia results (as reported, for example, in a letter from Sachs to General Watson on May 15, 1940) showing that the carbon absorption was appreciably lower than had been previously thought and that the probability of carbon being satisfactory as a moderator was therefore considerable. Sachs was also active in looking into the question of uranium ore supply. On June 1, 1940, Sachs, Briggs, and Urey met with Admiral Bowen to discuss approaching officials of the Union Miniere of the Belgian Congo. Such an approach was made shortly afterwards by Sachs.
In fact Edgar Sengier, head of Union Miniere, had already ordered a shipment of 1,200 tons of high-grade ore from the Shinkolobwe stockpile in the Congo, via Portuguese West Africa to New York. Oddly enough at the time the U.S. government was not interesting in this stockpile, but it was 'discovered' when Standard Oil, working on the centrifuge process, opened negotiations. Thus, by an odd series of events, the U.S. found enough uranium ore, just when they needed it.
The general status of the problem was discussed by a special advisory group called together by Briggs at the National Bureau of Standards on June 15, 1940. This meeting was attended by Briggs, Urey, Merle Anthony Tuve, Wigner, Breit, Fermi, Szilárd, and Pegram. After full discussion, the recommendation of the group to the Uranium Committee was that funds should be sought to support research on the uranium-carbon experiment along two lines:-
Further measurements of the nuclear constants involved in the proposed type of reaction.
Experiments with amounts of uranium and carbon equal to about 1/5th to 1/4th of the amount that could be estimated as the minimum in which a chain reaction would sustain itself.
"It was estimated that about $40,000 would be necessary for further measurements of the fundamental constants and that approximately $100,000 worth of metallic uranium and pure graphite would be needed for the intermediate experiment".
(Quote from memorandum of Pegram to Briggs, dated August 14, 1940)
In fact as far as I can see this money was used to purchase 4 tons of pure graphite and 50 tons of uranium oxide. The same meeting also decided that any large-scale uranium-carbon experiment should be supervised by the Army and Navy at one of their proving grounds. They also decided that isotope separation work should continue in the universities, and not on a secret basis.
As the Nazis were over running Belgium and looking towards France, there was increasing pressure for a research program with improved financial support and a more flexible organisation. The Uranium Committee had done its job, it had determined that the focus should be on both isotope separation and the chain reaction.
National Defence Research Committee
Before any decisions made at the last meeting of the Uranium Committee could be put into effect, the organisation of the National Defence Research Committee (NDRC) was announced in June 1, 1940, and President Roosevelt gave instructions that the Uranium Committee should be reconstituted as a subcommittee of the NDRC, reporting to Vannevar Bush (chairman, NDRC). The membership of this reconstituted Uranium Committee (or Section) was as follows: Briggs (Chairman), Pegram, Urey, Beams, Tuve, and Ross Gunn. In September 1941 Gunn and Tuve stood down, and the Committee was enlarged with the introduction of Breit, Samuel King Allison, Edward Uhler Condon, Henry DeWolf Smyth, and Lloyd Smith. On authorisation from Briggs, Breit consulted Wigner and Teller frequently although they were not members of the committee.
The National Defence Research Committee, with Vannevar Bush at its head, reorganised the Uranium Committee into a scientific body and eliminated military membership. Not dependent on the military for funds, as the Uranium Committee had been, the National Defence Research Committee would have more influence and more direct access to money for nuclear research. In the interest of security, Bush barred foreign-born scientists from committee membership and blocked the further publication of articles on uranium research. Retaining program responsibilities for uranium research in the new organisation as setup (among the National Defence Research Committee’s early priorities were studies also on radar, proximity fuses, and anti-submarine warfare), the Uranium Committee recommended that isotope separation methods and the chain reaction work continue to receive funding for the remainder of 1940. Bush approved the plan and allocated the funds.
Through to December 1941, a number of contracts were let. Their number and total amount grew gradually. Urey began to work on isotope separation by the centrifuge method under a Navy contract in the fall of 1940. Other contracts were granted to Columbia University, Princeton University, Standard Oil Development Company, Cornell University, Carnegie Institution of Washington, University of Minnesota, Iowa State College, John Hopkins University, National Bureau of Standards, University of Virginia, University of Chicago, and University of California in the course of the winter and spring of 1940-1941, until by November 1941 the total number of projects approved was sixteen, totalling about $300,000 ($100,000 for isotope separation and $140,000 for the chain reaction).
The Uranium Committee as formed in the summer of 1940 continued substantially unchanged until the summer of 1941. At that time the main committee was somewhat enlarged and subcommittees formed on isotope separation (Urey), theoretical aspects (Fermi, Szilárd), power production (Fermi, Szilárd) and heavy-water (Urey). It was thereafter called the Uranium Section or the S-l Section of NDRC.
Even today there are still inconsistencies across different 'official' histories leading up to the Manhattan Project. Some reports mention these 'subcommittees', others don't. In some reports it is mentioned that Uranium Committee became a Section (S-1) in the summer 1941. It is said that dropping the word uranium was, at least in part, for security reasons. Other reports mention that in June 1941 Roosevelt created the Office of Scientific Research and Development (OSRD). The NDRC fell under the new OSRD, and Vannevar Bush became its director, with James Bryant Conant as head of NDRC. The Uranium Committee became one of the 'sections' under the OSRD, however it was only in January 1942 that the section was reorganised and renamed Section 'S-1'. It was in fact removed from the NDRC and made an independent organisation within the OSRD. By June 1942 the chair of Section S-1 increasingly had the task of managing the relationship between OSRD and what was fast becoming an army project.
We have to remember that Germany invaded Norway in early April, 1940, securing control of the Norsk Hydro plant, the only large facility in the world producing heavy-water (deuterium oxide). This plant was sabotaged by the Norwegians to prevent the Germans acquiring the heave water. Harold Urey discovered the isotope deuterium in 1931 and was later able to concentrate it in water. Heavy-water is an alternative neutron moderator to graphite. Because they do not require uranium enrichment, heavy-water reactors are more of a concern for nuclear proliferation. The breeding and extraction of plutonium can be a relatively rapid and cheap route to building a nuclear weapon, as chemical separation of plutonium from fuel is easier than isotopic separation of uranium-235 from natural uranium.
In the spring of 1941, Briggs, feeling that an impartial review of the problem was desirable, requested Bush to appoint a review committee. Bush then formally requested Frank Baldwin Jewett, president of the National Academy of Sciences, to appoint such a committee. Jewett complied, appointing Compton (chairman), William David Coolidge, Lawrence, John Clarke Slater, John Hasbrouck Van Vleck, and Bancroft Gherardi Jr. This committee was instructed to evaluate the military importance of the uranium problem and to recommend the level of expenditure at which the problem should be investigated.
Again there are substantial discrepancies in different 'histories' about the members of this review. Other membership list don't mention Slater and Gerard Jr. as members, but do mention Oliver Ellsworth Buckley, Lewis Warrington Chubb, Warren Kendall Lewis, George Bogdanovich Kistiakowsky, and Robert Sanderson Mulliken.
Arthur Holly Compton (1892-1962) won the Nobel Prize in Physics in 1927 for the discovery of the Compton effect, thus demonstrating the particle nature of electro-magnetic radiation. He was leader of the Metallurgical Laboratory in Chicago, and later was in charge of plutonium production for the bomb.
Ernest Orlando Lawrence (1901-1958) won the Nobel Prize in Physics in 1939 for the invention of the cyclotron, and was responsible for isotope separation and uranium enrichment with the calutrons.
Pegram summarised progress in a report dated February 15, 1941 showing that the main progress had been made on understanding the slowing down of neutrons in graphite, on the number of neutrons emitted in fission, and on the design and size of lattices.
The slowing down of neutrons in graphite was investigated by studying the intensity of activation of various metal foils (rhodium, indium, iodine) placed at various positions inside a rectangular graphite column. With a source of neutrons placed at the bottom and using cadmium screens (cadmium having a very large absorption cross-section for thermal neutrons) the effects of resonance and thermal neutrons could be measured separately. These results, coupled with theoretical studies of the diffusion of thermal neutrons, laid a basis for future calculations of the number of thermal and resonance neutrons to be found at any point in a graphite mass of given shape when a given neutron source is placed at a specified position within or near the graphite. The experiments on slowing down neutrons showed that high-energy neutrons such as those from fission were practically all reduced to thermal energies after passing through 40 cm or more of graphite. A piece of uranium placed in a region where thermal neutrons were present absorbed the thermal neutrons and as fission occurred re-emitted fast neutrons, which were easily distinguished from the thermal neutrons. By a series of measurements with and without uranium present and with various detectors and absorbers, it was possible to get a value for the constant 𝜼, the number of neutrons emitted per thermal neutron absorbed by uranium. This is not the number of neutrons emitted per fission ƙ, but is somewhat smaller than that number since not every absorption causes fission.
The Fission of Uranium
In the last paragraph we have introduced a number of new physical concepts that need clarification within the context of the time. Research on the physics and chemistry needed to build an atomic bomb was substantial and it is impossible to even imagine summarising it all on one webpage, so I've decided here and there to focus on one or other key topic, and the first topic is the work of Fermi on the fission of uranium.
Fermi received the 1938 Nobel Prize in Physics, and after receiving his prize he continued on to New York where he applied for permanent residency. In early 1939 he took up a post as professor of physics at Columbia University. He more or less started where he had left off, looking at the fission process of the uranium nucleus by neutron bombardment.
Hahn and Strassmann had found chemical evidence for the splitting of the uranium nucleus into two approximately equal parts. Meitner and Frisch had suggested that the release of energy could be as much as 200 MeV. Fermi and his team started by looking at the fragments using an ionisation chamber. They coated one of the plates with a thin layer of uranium oxide, and they could immediately see a large number of small pulses from the alpha particles of uranium. But when exposed to the bombardment of neutrons from the cyclotron or Radon-Beryllium (Rn-Be) source very large pulses were detected.
The cyclotron was developed at Berkeley by Ernest O. Lawrence in the period 1931, and by 1936 John Ray Dunning and Herbert Lawrence Anderson had built one at Columbia University.
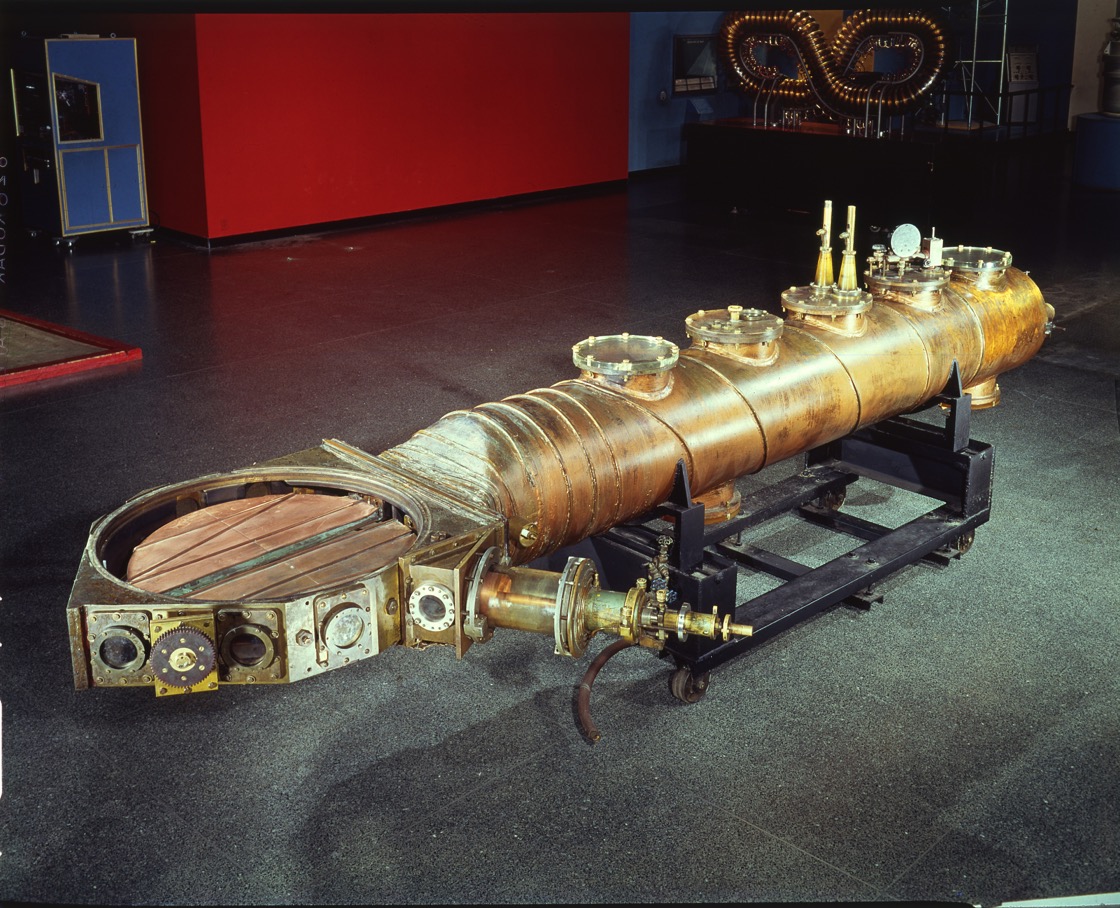
This is what people called the 'atom smasher'. This machine produced an energetic beam of protons which then struck a stationary metal target to produce a secondary neutron beam. In this sense it was an alternative to the 'natural' Ra-Be neutron source. This machine was used to show that it was uranium-235 that was the most readily fissile component of uranium. What we see above is the core component of the cyclotron which was decommissioned in 1965 and given to the Smithsonian Institute for restoration and display. The other component of the cyclotron were the large electro-magnets. Below we have Dunning (left), Fermi (centre) and Mitchell (right) standing in front of the magnets housed in the basement of Pupin Hall in Columbia University.
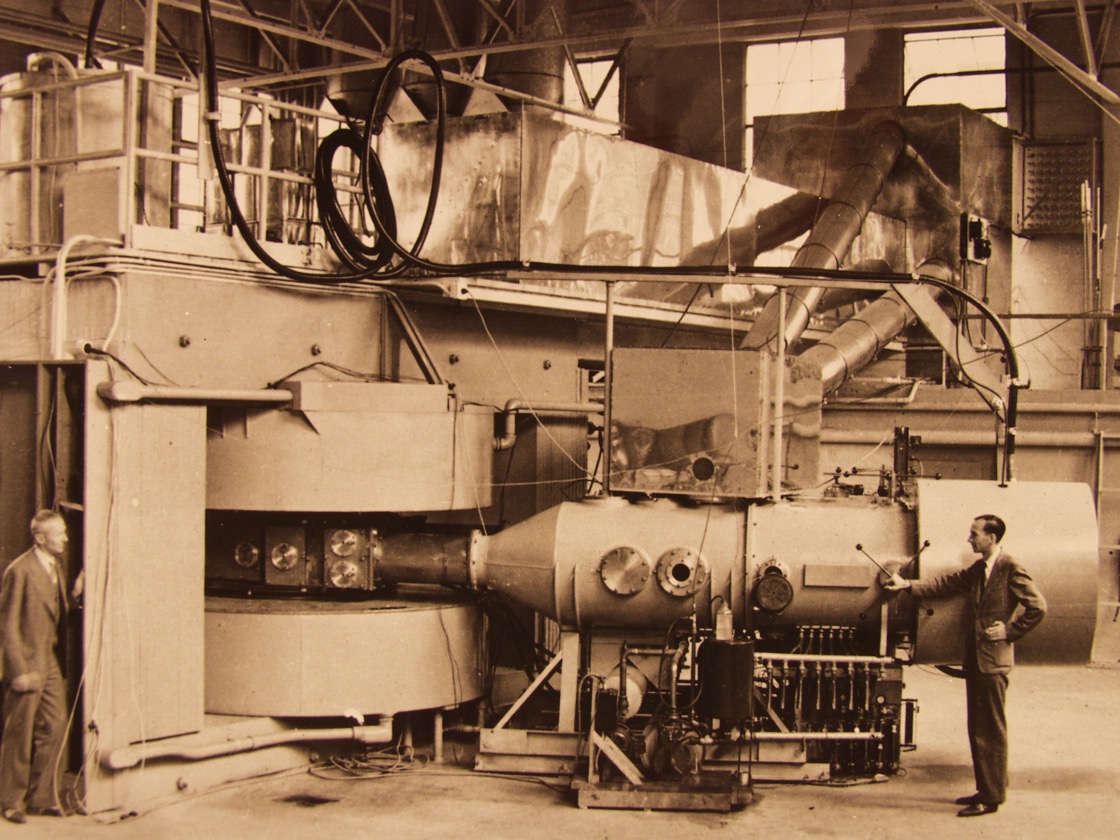
In 2006 it was finally decided to remove the 30 ton magnets for scrap.

Below is a photograph of the large cyclotron at the Lawrence Radiation Laboratory, showing how the cyclotron cavity, etc. would be inserted between the poles of the electro-magnet.
Now back to fission fragments. Looking at the ratio of the pulses they saw in their ionisation chamber they were able to estimate that the energies of the fragments of uranium ranged up to about 90 MeV, and given that the expectation was a total energy release of about 200 MeV this suggested that there were two fragments one somewhat more energetic and the other less so. At the same time Frisch had cabled Bohr with the same results. Fermi moved onto the measurement of the 'cross-section' of the fission process for neutrons of different energies. They coated the inside of the ionisation chamber with a very thin layer of uranium oxide, and bombarded it with a known number of neutrons from an Rn-Be source of known intensity placed inside a paraffin block. Thermal neutrons were isolated by measurements with and without a cadmium absorber. They estimated the cross-section of thermal neutron for the fission process as about 2 x 10-24 cm2 (compared to 0.1 x 10-24 cm2 for fast neutrons). Further experiments suggested that the efficiency of slow neutrons for the fission process followed an inverse law of the velocity (1/v). This appeared inconsistent with the known fact that in uranium there was a sharp resonance for slow neutrons at about 25 eV which did not lead to fission but to the formation of uranium-239. It was Bohr who suggested that the fission might not be occurring in uranium-238 but in uranium-235 (present in natural occurring uranium with a concentration of about 0.7%).
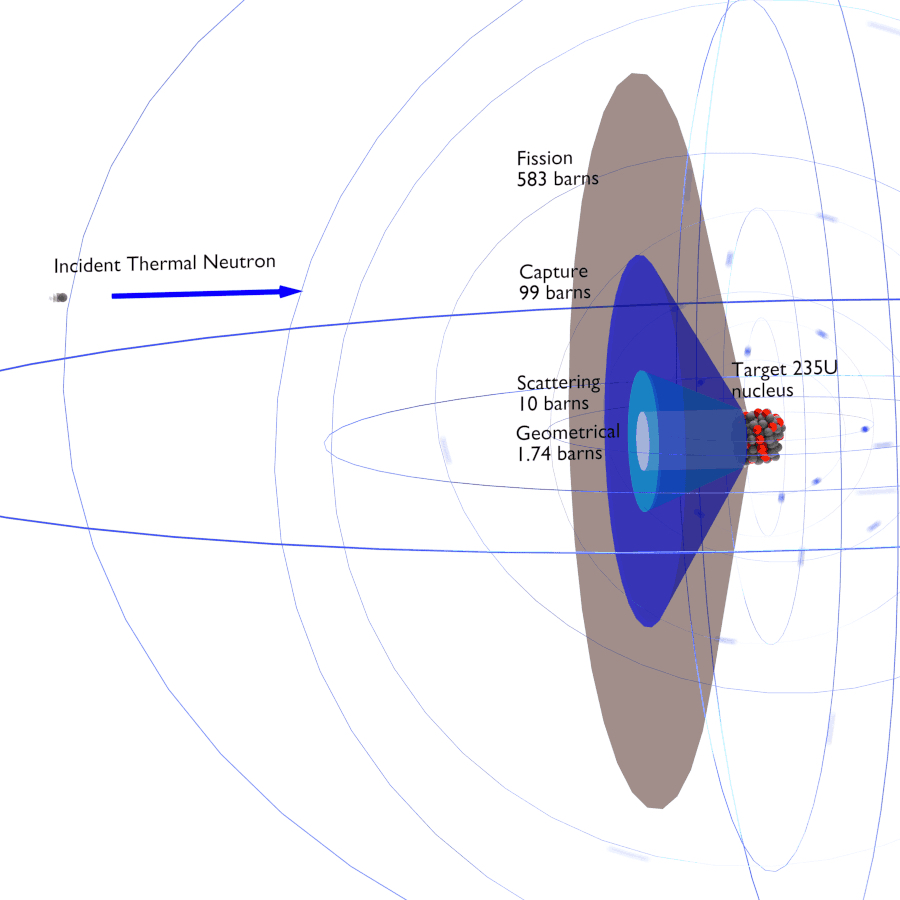
It is worthwhile mentioning here that neutrons are neutral particles that travel in straight lines, and are deviated only by collision with a nucleus (not with the electric field around the nucleus). They can be deviated or absorbed. The 'barn' is a unit for measurements of cross-sections.
Above we have a very effective presentation of the meaning of 'cross-section'. It is an effective area that represents the likelihood of a certain type of interaction (a kind of collision). The cross-section of a nucleus represents the probability of a particular type of nuclear reaction, so it is in many ways a 'characteristic target area', but it can vary significantly depending upon the type and velocity of the 'projectile' (particle). The total cross-section is made up of several different types of interaction, i.e. elastic scattering (n,n), inelastic scattering (n,n'), radiative absorption (n,𝜸), and fission (n/f).
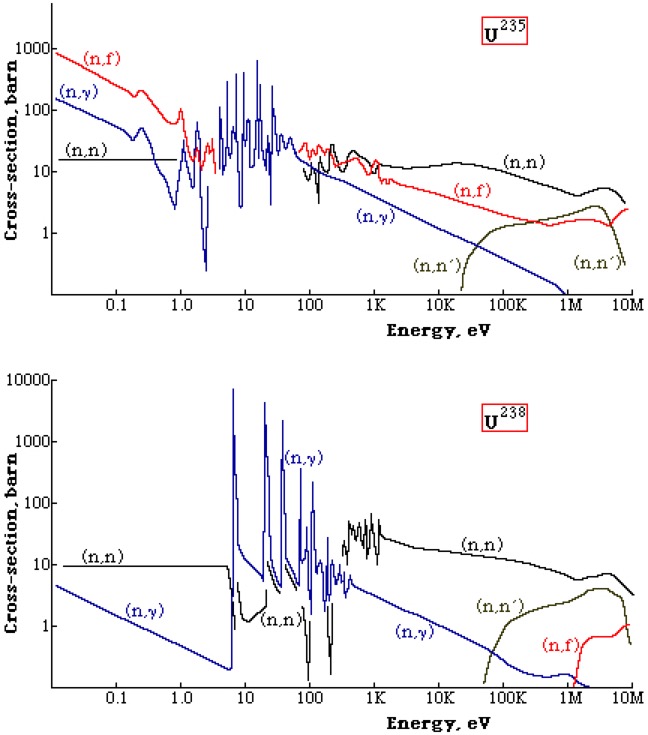
What Fermi was interested in was the possibility for neutrons to be absorbed and to subsequently emit other neutrons that would lead to a self-sustaining or 'chain reaction'. The reality is that such a reaction cannot occur in natural uranium, and we can see this in the neutron cross-sections for uranium-235 and uranium-238. Uranium-235 will fission (n,f) at all energies (we call this a fissile material). Uranium-238 has a lower threshold for fission (n,f) at about 1 MeV. There is a very strong resonance capture of neutrons (n,𝜸) in the energy range 10-100 eV, in particular for uranium-238. The fission neutron energy spectrum peaks at around 1 MeV and at this energy the inelastic cross-section (n,n') for uranium-238 exceeds the fission cross-section, essentially preventing fission for uranium-238. The fission cross-section is small for the fission neutrons and therefore the neutrons are absorbed by other processes, thus natural uranium cannot sustain a fission chain reaction.
The classical way to view this is to take 100 fission neutrons, 98 will be captured in uranium-238 and only 8 of those captured will result in a fission. The remaining 2 neutrons will cause fission in uranium-235, and each fission will produce 2-3 neutrons. So we started with 100 neutrons, and now in the second generation we have a maximum of 25, thus no chain reaction.
How to get around this? Firstly we can enrich the uranium with more uranium-235. A 50-50 mix will easily sustain a chain reaction with most of the fission events occurring in uranium-235 in the energy range 03-2.0 keV. This is called a 'fast reactor'. Or we can mix natural uranium with a material (a moderator) that slows down the neutrons without absorption, as we get to the low energies the fission cross-section of uranium-235 increases rapidly. Now the fissions are induced by thermal neutrons (0.025 eV), hence the name 'thermal reactor'.
The team at Columbia went on to use the cyclotron and collect the recoil fragments on Cellophane foils which were later measured for their activation. It was at this point that whilst the team pursued the details of the fission process, Fermi with Anderson focussed on neutron emission from the splitting of the uranium nucleus.
They knew neutrons were emitted in the process of fission, but did they emerge from the excited fragments or at the instant of fission? They started by placing the neutron source in the centre of a spherical bulb immersed in a large tank of water. They used the activation of rhodium foils placed at different locations in the tank to take measurements with and without uranium oxide inside the bulb. All the neutrons in the water are slow, and they found that there was a 6% increase with the uranium, corresponding to a yield of about 2 neutrons emitted for each neutron captured. These experiments took place independently of those of Von Halban, Joliot and Kowarski in Paris.
What we tend to see is an almost 'linear' and highly logical picture of the key moments in the development of nuclear fission, and forget all the other experiments that are needed to understand the physics of the fission process. One of the problems with fission by slow neutrons is that uranium-238 can simply capture the neutron giving rise to the radioactive isotope uranium-239. This process competes with fission, thus absorbing neutrons that are needed to sustain a chain reaction. This appeared on the surface to be a simple question, but it turned out that neutron capture by uranium-238 leads, through neptunium, to the production of plutonium.
Meitner, Hahn and Strassmann had already published on the resonance capture process with a sharp absorption at about 25 eV (a cross-section of about 1200 x 10-24 cm2). But there was no information about the width of the absorption band or the cross-section for thermal neutrons. Fermi and Anderson found that the cross-section for the production of uranium-239 by thermal neutrons was only about 3.2 x 10-24 cm2 (it later turned out to be about 6 x 10-24 cm2) and the width of the resonance was determined at about 1 eV.
Resonance capture involves the creation of an excited state of a nucleus formed by the combination of the incident particle and the target nucleus. This occurs when the energy of the particle is near to a certain defined energy value (usually low energies). Different peaks correspond to different compound states of the nucleus.
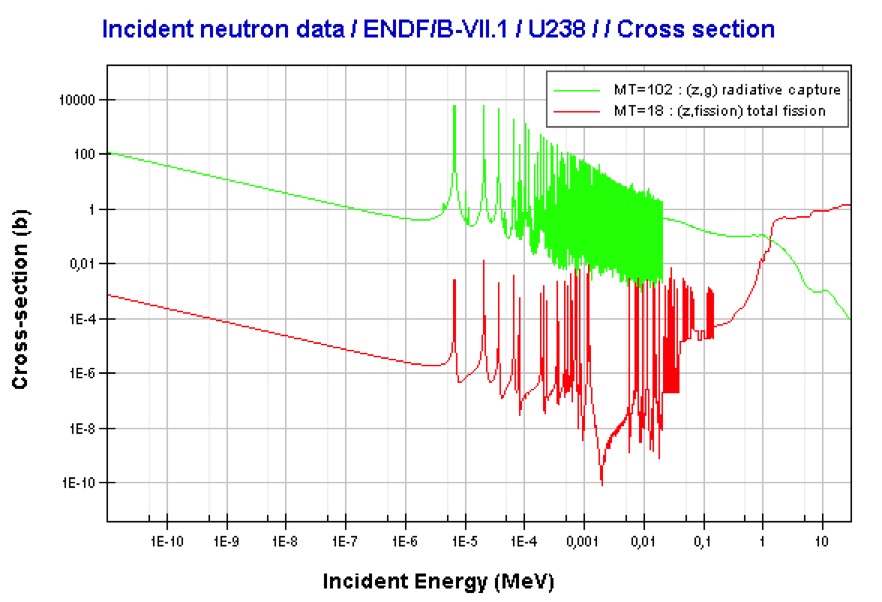
Uranium-238 (above) is a fissionable isotope but not a fissile isotope. It is not capable of undergoing fission reactions after absorbing thermal neutrons. On the other hand it can be fissioned by fast neutrons with energies > 1 MeV. Uranium-238 is not able to sustain a nuclear fission chain reaction because too many of the neutrons produced by fission have lower energies than the origin neutron. We say that uranium-238 is a fertile isotope in that it's radiative capture of a neutron leads to the formation of fissile plutonium-239.
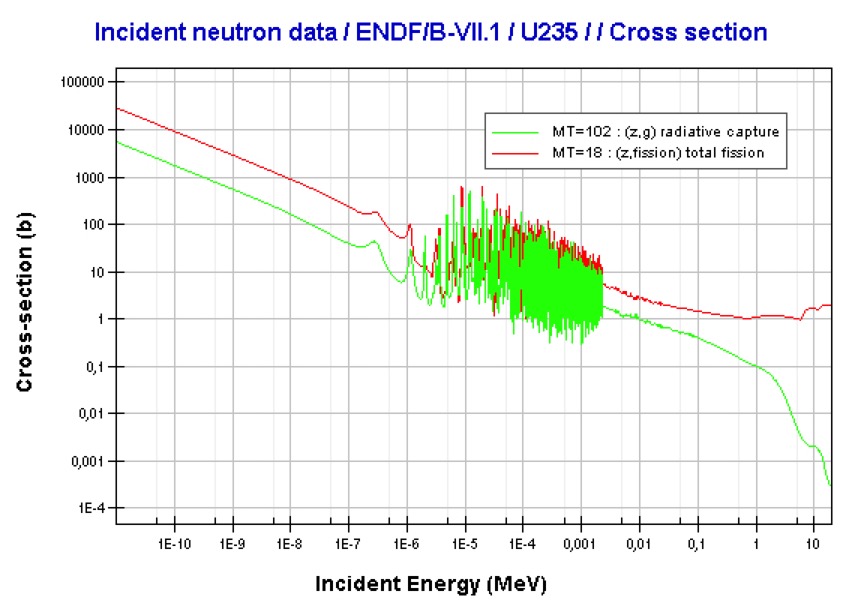
Uranium-235 is a fissile isotope and it has a large fission cross-section for thermal neutrons, and a very small cross-section for fission with fast neutrons. Most of the absorption reactions result in fission, but about 15% are radiative capture forming uranium-236.
About the same time Leo Szilárd was also in Columbia University. He had obtained about 20 kg of uranium oxide and they placed it around a Ra-Be source and all immersed in a bath of 10% manganese solution. This experiment confirmed that more neutrons were emitted by uranium than it absorbed. They went on to conclude that "a nuclear chain reaction could be maintained in a system in which neutrons are slowed down without much adsorption until they reach thermal energies and are then mostly absorbed by uranium rather than by another element". There was an issue, in that in a system composed essentially of uranium and hydrogen (i.e. water) they would need to produce more neutrons from uranium than were absorbed by the uranium and hydrogen together. Too much hydrogen would prevent a chain reaction. So as of July 1939 their initial conclusion was that even with an 'optimum' concentration of hydrogen it was uncertain whether neutron production would exceed the total neutron absorption. At this time they thought that they would need to lump the uranium together to reduce the losses due to resonance absorption, and that the absorption of hydrogen in water would make it unusable for slowing down neutrons in a chain reaction.
Fermi and Szilárd did not get on that well, but it was Szilárd that became convinced that with graphite to slow down the neutrons the chain reaction was a certainty. He and Eugene Paul Wigner convinced Einstein to write his famous letter to President Roosevelt. Fermi and George Braxton Pegram (Dean of Columbia) had tried to alert the authorities to implications of atomic energy, but perhaps their language had been too cautious. In any case Einstein's letter led in early 1940 to a first government grant of $6,000 for buying granite to determine its neutron absorption.
An interesting aside Anderson's doctorate thesis was on resonance absorption in uranium. Szilárd was concerned that uranium research should be kept a secret, and he wanted everyone to withhold publication. But he needed a first example, and Anderson's thesis was that first example. He got his doctorate but the thesis was deposited and not published. Future authors in the field were also asked to withhold publication. Results funded by the U.S. government were marked 'secret'.
With the $6,000 funding Szilárd was able to buy 1½ ton of graphite all nicely presented as bricks. The bricks were placed in a neat pile with holes left for inserting the rhodium foils for neutron activation. Rhodium activation has a half-life of 44 seconds, so the foils had to be pulled and measured quickly. Fermi loved the process of irradiating the foils, extracting them and rushing down the corridor, and watching the scaling circuit light up on the Geiger counter. The results were that graphite was the obvious choice to slow down neutrons. The basic idea was that fast neutrons collide many times with graphite nuclei, losing energy until they reach thermal energy. Then they diffuse through the graphite without losing further energy, and then they are finally absorbed. The neutrons are slowed down more slowly than in water, but once they reach thermal energies the neutrons diffuse longer and reach greater distances from the source. This meant that it would be easier to physically separate thermal neutrons from higher energy neutrons. The general model describing this neutron diffusion is called the 'age theory'.
I hate to do this but the below diagram shows how Fermi 'saw' the neutron slowing down process (forget the maths). We see that a fast neutron will travel quite a distance in a quasi-straight line losing energy in a few collisions. As it becomes a thermalised neutron it will start to meander around travelling quite a distance but not in a straight line (it can even turn back on itself). Finally it will be absorbed.
Another topic that attracted Fermi, and forced him to become a radio-chemist for a short while, was the so-called 'branching ratios' in the fission of uranium-235. A large number of radioactive series were found among the fission products of uranium, and the branching ratio is the probability that a specific radioactive series occurs. With this experiment Fermi and Anderson used the expertise of the radio-chemist Aristid von Grosse. They were back using the cyclotron and by that time they had determined that slow neutron fission took place in the uranium-235. As far as I can tell the technique was to create a kind of standard measurement configuration with a uranium solution inside a paraffin block placed in a fixed position near the cyclotron. The configuration was designed to ensure that only slow neutrons were producing fissions in the uranium-235. Then a known quantity of each element was added as a carrier to the uranium solution, and then after irradiation the solution was purified and separated. A weighted fraction of the added amount was painted onto a thin aluminium strip and was wrapped around a thin walled silver-glass counter. This enabled the team to make comparative measurements in a highly standardised way, and then to calculate the branching ratio, the fraction of fissions giving rise to a particular radioactive series. From what I can understand the key task was to separate the different elemental series studied, i.e. iodine, antimony, barium, and zirconium. I must admit after reading the original research papers I could not extract a clear set of results, however the measurements were later repeated in an extensive way in the Metallurgical Laboratory in the University of Chicago.
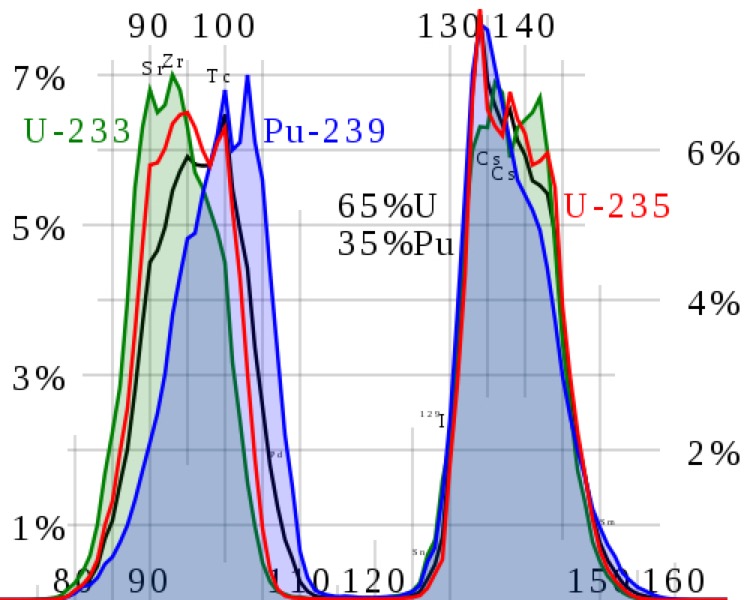
Above we can see the fission fragment yield for different nuclei. The most probable fragment masses are around krypton-95 and barium-137. For example as uranium-235 fissions the nucleus splits in to two smaller nuclei accompanied by a few neutrons. Most fission fragments are unstable and will decay. Most of the energy released in a fission is in the form of the kinetic energy of these fission fragments. As the fission fragments move through material they ionise the surrounding atoms, and gradually slow down. As the positive ions re-bond with the free electrons they release the excess energy in the form of heat.
Now that the Columbia team had a significant supply of graphite they could turn to the problem of precisely measuring the average number of neutrons produced by uranium upon the capture of a thermal neutron. Up to then the measurements were made with considerable uncertainty. They created a tall column of graphite with a Rn-Be source at one end and a window at about 70 cm from the source. The key was that at this distance the intensity of neutrons at a resonance energy was very low compared to the intensity of thermal neutrons. So uranium inserted in the window would absorb only thermal neutrons. After quite a complex set of side measurements, corrections, etc. the average number of neutrons produced per thermal neutron captured by uranium was 1.73.
All the above documented research focussed on understanding better the chain reaction as a way to produce power, not a bomb.
On 30 June, 1941 Fermi produced a kind of summary on the problems that might arise during the release of atomic energy. The starting point was that if a chain reaction was used as a radiation source, or as a way to create isotopes or produce new elements, then there would be a problem to dissipate the large amount of energy released during the reaction. In fact energy dissipation would probably be the limiting factor in how to exploit a chain reaction.
At that moment in time the idea was to use uranium without any separation of the isotopes, even if the separation of uranium-235 and of plutonium was already being studied and achieved albeit on a very small scale.
The basic idea was to assembly lumps of uranium metal or oxide in a lattice array throughout a mass of a light element (carbon, beryllium, heavy-water) used to slow the neutrons produced in the fission process and enable them to react again with the uranium reproducing new fission processes. The energy produced would be in the form of kinetic energy of the fission fragments, the beta- and gamma-rays, and the kinetic energy of the neutrons. All these energy sources will in the end produce heat, but it is not clear where this heat would be deposited (e.g. inside the materials or outside). Fermi estimated that most of the energy would be released by the kinetic energy of the fission fragments, and that most of the energy would be converted in to heat in the uranium lumps (perhaps as much as 90%). The reaction would need to be controlled to allow heat to be transported away from the lumps. More power could be generated if the heat was removed artificially. However most chemical elements that could be used to remove the heat have a high absorption for neutrons, and would likely stop the reaction. Probably the best way to remove heat is by using a fluid (gas or liquid) that would pass through channels or pipes. The heat could be used to vaporise the liquid, and the chain reaction would then become a boiler.
Gases could be helium or carbon dioxide (oxygen was ruled because of its poor chemical properties). Liquids could be deuterium based or possible liquid bismuth. Very large volumes of gases would be needed, liquid would require smaller volumes. Vaporised liquids in the form of heavy-water or some carbon fluorides had some promise.
The reaction would need to be controlled. However the change in the reaction could be exponential in both senses. The neutrons are emitted almost instantaneously and the speed in which the control would be applied would need to be of the order of 1/1000th of a second. Fortunately a reaction using delayed neutrons would require a speed of control of the order of 10 seconds. It may even be that the reaction is auto-controlled in that the rise in temperature might produce an increased loss of neutrons, and regulation could be achieved by managing the reflection properties of the materials.
Fermi concluded with the warning that the radiation emitted would be extremely dangerous, and a thick water screen would be needed.
It would appear that it was in spring 1941 that the military importance of the uranium work started to take precedence, despite the fact that most scientists doubted that atomic energy would come in time to affect the war. Some scientists such as Szilárd were convinced that an atomic bomb was feasible. In May 1941 there was a first cautious report on the difficulties in separating a sufficient amount of uranium-235 for a bomb. A report in July 1941 revealed the results on plutonium and mentioned the possibility of a bomb.
We are now in October 1941 and there was still a note of caution "separated uranium isotopes seem to bear out the assumption that fissions observed with neutrons of energy below 1 MeV are due to uranium-235". Fermi's calculation of the critical mass was 130 kg, but varied from 20 kg through to more than 1 ton. He also noted that the critical mass could be reduced by surrounding the 'reacting unit' with a reflector of very dense and think material. Nevertheless we are approaching the climax of the work in Columbia University, with a long series experiments on different types of lattices of uranium oxide lumps embedded in graphite. Initially the tests were inconclusive, the purity of the uranium and the graphite was questioned, and the density of the oxide was considered too low. One particular problem was the leakage of neutrons from the structure, and the idea that a very large structure was needed. Fermi in his attempts to use a smaller structure, invented what they called an 'exponential experiment'. This consisted of a rectangular column made up of a uranium-graphite lattice, and a neutron source placed at the bottom. The neutron density decreases exponentially along the length of the column, and is a function of the leakage along the column. A new grant of $40,000 allowed the team to build a lattice column about 2 metres square and nearly 4 metres high. With this they found a multiplication factor (or 'reproduction factor ƙ') of 0.87, which despite being below 1 was seen as a very positive result. Everyone felt that by improving the purity, the geometry, and the density of the uranium they could exceed a multiplication factor of 1.
In fact at this moment in time no one appeared able to manufacture either non-pyrophoric uranium power (does not ignite spontaneously) or pure uranium ingots. In fact all isotopes of uranium are pyrophoric, but much depends upon the rate of surface oxidation balanced against the heat loss to the surroundings. However this feature is not the reason depleted uranium is used as kinetic energy penetrators. It is used because its density makes a bigger impact, and it is the abrupt slowing down upon impact that turns the penetrator white hot. In fact the tip is vaporised, and the projectile stays sharp because its alloyed with titanium forming a large metallic crystal. When it finally penetrates into the vehicle its a white-hot gas that ignites on-board annunciation and fuel. Plus depleted uranium is almost free.
In addition, at the it was still problematic to obtain graphite with a low boron content. In fact it would take another 2 years to develop thermal and gas extraction purification techniques so as to obtain so-called nuclear-grade graphite. An order placed in May 1941 for 40 tons of graphite certainly helped.
In late 1941 Fermi made a masterful review of the chain reaction. The lattice consists of uranium lumps (at this time it was natural or un-enriched uranium) disposed in a mass of graphite. Fast neutrons are produced by the uranium lumps and are slowed down to thermal energies by collisions, primarily with carbon atoms. After reaching thermal energies the neutrons keep diffusing through the mass until absorbed by uranium or carbon. Some of the neutrons absorbed by the uranium atoms produce fission processes in which fast neutrons are emitted, and a new cycle of slowing down and absorption begins again. Fermi termed this a 'generation', and a key descriptor in the sequence of generations is the 'reproduction factor ƙ' defined as "the average number of new fast neutrons produced in an infinite lattice by one original fast neutron in the first generation". With ƙ>1 the series of generations expands exponentially. In a finite lattice ƙ is limited by the leakage of neutrons. Fermi calculated that a uranium lump would need to be surrounded by 327 cm of graphite for there to be no thermal neutron leakage.
Below I've included two diagrams of the uranium-235 chain reaction as we understand it today.
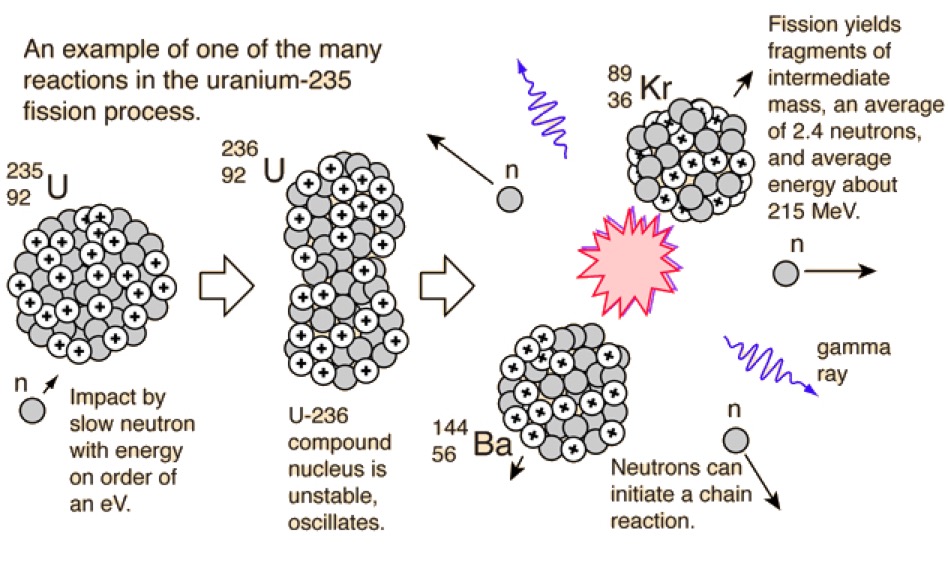
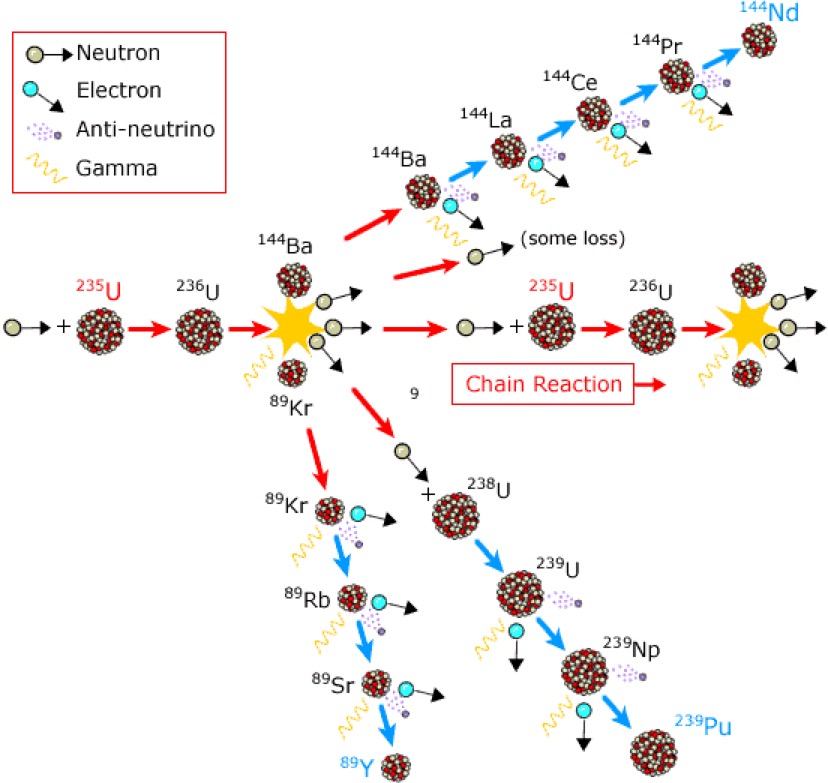
The team in Columbia then performed experiments to ascertain whether a lattice of uranium oxide lumps embedded in graphite could produce a 'divergent' chain reaction if the dimensions were sufficiently large. You will remember the uranium and graphite column. It was placed on a 3 metre square paraffin base about 30 cm high. A Ra-Be source was placed inside the paraffin base and intensity measurements were taken in different places in the column. It was in this experiment that a ƙ of 0.87 was found. They also found that ƙ was smaller at high temperatures than at low temperatures.
These were the last measurements made in Columbia University before the move to Chicago. The move was decided in January 1942. We have to remember that what had slowly emerged was the idea that a chain reaction could also be used to produce plutonium, as a competitor to uranium-235, for a possible atomic bomb. It was on the December 6, 1941 that it was decided to make an 'all out' effort on understanding the chain reaction. The next day Pearl Harbor was attacked, the U.S. declared war on Japan, Germany, and Italy, and Fermi became an enemy alien. It was under these circumstances that the Metallurgical Laboratory at the University of Chicago was established (February 1942). It was decided that they would study the chain reaction with natural uranium as a way to produce plutonium. Columbia University was already committed to two major projects, uranium separation headed by Harold Clayton Urey and the cyclotron project with John Ray Dunning. Fermi was an enemy alien, so Arthur Holly Compton was made project director for the work on the pile. He decided on Chicago, and Fermi and his team had to move. Fermi became submerged in an influx of scientists working on the chemistry of fission products and plutonium, on plant design, on the fabrication of uranium metal, and even specialists on the effect of radiation. And all this within am increasingly strict framework of military security.
We will continue to follow the work of Fermi and his team at Chicago in a later section dedicated to the Chicago Pile-1. In addition to the work going on in Columbia there were measurements being performed on alternative moderators to graphite. Samual King Allison was in the University of Chicago looking at beryllium as an alternative moderator, and Urey was still interested in heavy hydrogen (heavy-water) as another alternative moderator.
To close this parenthesis on Fermi in Columbia University on a lighter note. There are a number of stories about the tunnels under Columbia University, or as one writer put it "the abode of history, crime, and particle accelerators". Over the years students had tried to investigate what was behind the 'No Entry' in the basement of the different buildings. There is even one tunnel that was used for transporting coal for the heating. And the tunnels were exploited by both the students and the police in the 1968 riots. As we have seen the basement of the Pupin Hall was home to one of the worlds first cyclotrons and was thus off-limits for radiation hazard reasons. As one writer noted, now the basement corridors have been refurbished and the traces of Fermi, et. al. have completely disappeared. Something to ponder on!
Progress is Made: but Towards What?

Chicago Pile-1 (CP-1), the site of the first human-made, self-sustaining nuclear reaction. It consisted of a large, monolithic pile of uranium pellets and graphite blocks, with cadmium, indium, and silver control rods, but no radiation shield and cooling system. This is in fact just one of the multiple builds and re-builds of the same pile.
By the time Fermi moved to Chicago I think he had a fairly clear idea what he was planning on doing. He was going to try and build an atomic 'pile'. The fundamental idea of a “pile” was a lattice structure with uranium concentrated in lumps regularly distributed in a matrix of (graphite) moderator. If the uranium and the moderator are mixed homogeneously, the neutrons on average will lose energy in small steps between passages through the uranium. So in the course of their reduction to thermal velocity the chance of passing through uranium at any given velocity, e.g. at a velocity corresponding to resonance absorption, is great. But, if the uranium is in large lumps spaced at large intervals in the moderator, the amounts of energy lost by neutrons between passages from one lump of uranium to another will be large and the chance of reaching a uranium lump with energy just equal to the energy of resonance absorption is relatively small. Thus the chance of absorption by uranium-238 to produce uranium-239, compared to the chance of absorption as thermal neutrons to cause fission, may be reduced sufficiently to allow a chain reaction to take place. If one knew the exact values of the cross-sections of each uranium isotope for each type of absorption and every range of neutron speed, and had similar knowledge for the moderator, one could calculate the "optimum lattice", i.e. the best size, shape and spacing for the lumps of uranium in the matrix of moderator. Such data were only partially known, and as we have seen experiments were initiated at Columbia, and additional experiments were started at Princeton in Feburary 1941. The key was to try and measure the absorption of neutrons by uranium under conditions similar to those expected in a chain-reacting pile employing graphite as moderator.
During 1939 and 1940 most of the work done on isotope separation and the chain reaction pile was performed in university laboratories by academic scientists funded primarily by private foundations. While the U.S. federal government began supporting uranium research in 1940, the pace appeared too leisurely to the scientific community and failed to convince scientists that their work was high priority. Certainly few were more inclined to this view than Ernest O. Lawrence, director of the Radiation Laboratory at the University of California in Berkeley. Lawrence was among those who thought that it was merely a matter of time before the United States was drawn into WW II, and he wanted the government to mobilise its scientific forces as rapidly as possible.
Specifically what Lawrence had on his mind in early 1941 were experiments taking place in his own laboratory using samples produced in the cyclotron. Studies on uranium fission fragments by Edwin Mattison McMillan and Philip Hauge Abelson led to the chemical identification of element 93, neptunium, while research by Glenn Theodore Seaborg revealed that an isotope of neptunium decayed to yet another transuranium (man-made) element. In February 1941, Seaborg identified this as element 94, which he later named plutonium (a natural follow-on based upon the names of the planets, after Neptune came Pluto).
By May 1941, Seaborg had proven that plutonium-239 was 1.7 times as likely as uranium-235 to fission. This finding made the Fermi-Szilárd experiment more important than ever as it suggested the possibility of producing large amounts of the fissionable plutonium in a uranium pile using plentiful uranium-238 and then separating the different elements chemically. Surely this would be less expensive and simpler than building isotope-separation plants. Lawrence, demonstrating his characteristic energy and impatience, launched a campaign to speed up uranium research. He began by proposing to convert his smaller cyclotron into a spectrograph to produce uranium-235. Since both the cyclotron and the spectrograph used a vacuum chamber and an electro-magnet, this conversion was relatively uncomplicated. Lawrence then took his case to Karl T. Compton and Alfred Lee Loomis at Harvard University, both doing radar work for the National Defense Research Committee. Infected by Lawrence’s enthusiasm, Compton forwarded Lawrence’s optimistic assessment on uranium research to Bush, warning that Germany was undoubtedly making progress and that Briggs and the Uranium Committee were moving too slowly. Compton also noted that the British were ahead of their American colleagues, even though, in his opinion, they were inferior in both numbers and ability.
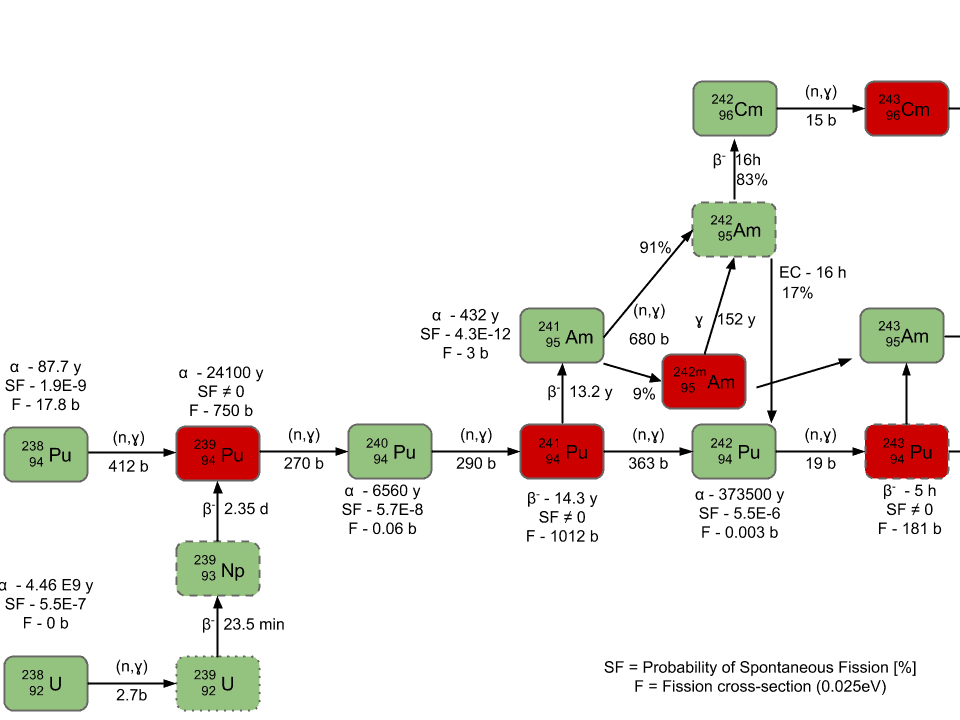
Chain of the transuranic elements. Neptunium (Np) was first synthesised by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Plutonium (Pu) was first produced and isolated in 1940 by Glenn T. Seaborg, Joseph W. Kennedy, Edwin M. McMillan, and Arthur C. Wahl. Americium (Am) was first produced in 1944 by the group of Glenn T. Seaborg from Berkeley, California, at the Metallurgical Laboratory of the University of Chicago, a part of the Manhattan Project. Curium (Cm) was first intentionally produced and identified in July 1944 by the group of Glenn T. Seaborg at the University of California, Berkeley. Other elements, such as Californium (Cf), were produced and identified after WW II.
Some books mention plutonium as being man-made, or discovered, or synthesised, but few mention plutonium-244, the only naturally occurring plutonium isotope, with a half-life of 80.8 million years. It has been postulated that this isotope has existed since the creation of Earth about 4.5 billion years ago (all heavy elements were formed in supernova explosions). It should also be mentioned that uranium in the Earth’s crust is also producing naturally plutonium, albeit on the order of one part in 1011 in pitchblende. And it is also worthwhile mentioning that apart from its formation in today's nuclear reactors, plutonium was formed by the operation of naturally-occurring nuclear reactors in uranium deposits at Oklo in what is now Gabon, some two billion years ago. However, most of the plutonium in the Earth’s environment comes from weapons tests, which released about 3 tons of plutonium into the atmosphere. There exists also plutonium-245, plutonium-246, and plutonium-247, all with half-lives of a few days. In fact, plutonium has 23 known isotopes, ranging in mass from 228 to 247, and 9 isotopes have metastable states that usually last less than 1 second.
In discovering plutonium Seaborg's team actually discovered (or made) two different 'types' of plutonium. They discovered that plutonium had two different oxidation states, i.e. in reacting with other elements plutonium forms two ions by giving up either 4 or 6 electrons, and each oxidation state has a completely different chemical behaviour. On top of that by treating plutonium with certain reagents the oxidation state will change. Immediately a possible 'reduction-oxidation' process (Redox) was imagined that could be used to purity plutonium. Initially plutonium in the lower oxidation state could be precipitated. Then it could be oxidised to the higher state and remain in solution during a second precipitation. Then it could be reduced to the lower oxidation state, and the same routine continued. The first samples of plutonium were microscopic, but Seaborg and his team managed to develop an industrial scale chemical process from scratch by August 18, 1942 ( the scaling factor was nearly 1 to 1 billion). However, this feature was perhaps the least unusual of the characteristics that they would discover for plutonium. Physicists might love plutonium, but it's a chemists and engineers nightmare. They would discover that plutonium is in some ways just another metal, i.e. electro-positive, dissolves in mineral acids, but at the same time it's a poor conductor. It has twice the density of iron, and is incredibly sensitive to temperature, and can undergo dramatic length changes (>20%). In one of the phases it will expand 5 times faster than iron, yet in another phase whilst being heated it will contract. Before turning liquid it goes through 6 different crystal structures and phase changes, including three expansions and two contractions (there is a 7th phase that appears at high pressures). It can change phases quite suddenly, and when it melts to become a liquid it contracts, becoming more dense. In its liquid state it has a very high surface tension, and it has the greatest viscosity of any element. Yet with the addition of a few atoms of aluminium or gallium, plutonium becomes malleable and easily pressed into different shapes. And just to make life interesting when in its close-packed crystal phase, it actually has its lowest density. And I have not mentioned plutonium's chemical characteristics. Just as an example, plutonium acquires very rapidly a protective layer of plutonium oxide (like aluminium), and will corrode at a very, very slow rate. Except in moist air where it will corrode 200 times faster at room temperature and 100,000 times faster at 100°C. Plutonium ages by corrosion, but also from within by internal alpha-particle decay damage, and this damage makes plutonium noticeably warm to touch. And we should never forget that plutonium is pyrophoric above 500°C, but in powder form it can ignite at 150°C.
Americium-241, with a half-life of 432 years, was the first americium isotope to be isolated, and is still the one used today in many domestic smoke detectors, although they have been banned in some countries and are being replaced by smoke detectors using the photoelectric circuit.
In mid-2014 a plan was announced to extract americium-241 from the UK plutonium stockpile, much of it old. According to the U.K. National Nuclear Laboratory (NNL), about 250 kg of old civil plutonium (originally with about 10-14% plutonium-241) will yield 10 kg of americium-241. The European Space Agency is paying NNL to produce americium-241 for 10-watt (e) radioisotope thermoelectric generators (RTG’s) using very pure americium-241 recovered from old civil plutonium, as the isotope is much less expensive than plutonium-238 (now scarce). NASA has now decided to pay for the cost of domestic plutonium-238 processing, but the U.S. still has about 35 kg of plutonium-238 in reserve. Americium costs about $1500 per gram, whereas the U.S. stopped producing plutonium-238 in 1988 (and bought some 16.5 kg produced at Mayak) and the Russians have also stopped producing and selling it internationally. My understanding is that the Mars rover Curiosity was fuelled with 4.8 kg of plutonium-238, to produce 2 kW (th), the equivalent would have been around 15-20 kg of americium-241. Around 250 plutonium-powered pacemakers were manufactured, and 22 of them are still in service after 25 years (there were discontinued when it was found that they would not remain intact after cremation).
Ernest O. Lawrence bombarding uranium with neutrons discovered that the capture of fast neutrons by uranium-238 transmuted that isotope first into element-93 and then into element-94, which they named neptunium and plutonium, respectively. After further investigation of these transuranium elements, neither of which was then known to exist in nature, Lawrence's group concluded that plutonium had the same fission characteristics as uranium-235, i.e. it could be split by neutrons and would, in turn, release more neutrons. Uranium-238, hitherto regarded as worthless for energy purposes, was in fact a prime source. Furthermore, as there was reason to believe that chemical separation of plutonium from uranium might prove more practicable than isotopic separation of uranium-235 from uranium-238, chances that an atomic bomb based on a fast neutron chain reaction could be built were tremendously increased.
In internal communications uranium-235 was often referred to as “25”, and plutonium as “49”, for the element no. 94, isotope atomic number 239.
The review committee requested by Vannevar Bush met in May, 1941 and submitted a report. On the basis of this report and the oral exposition by Briggs before a meeting of the NDRC, an appropriation of $267,000 was approved by the NDRC at its meeting of July 18, 1941, and the probability that much larger expenditures would be necessary was indicated. Bush asked for a second report with emphasis on engineering aspects, and in order to meet this request Oliver Ellsworth Buckley of the Bell Telephone Laboratories and Lewis Warrington Chubb of the Westinghouse Electrical and Manufacturing Company were added to the committee. The second report was submitted by Coolidge. As a result of new measurements of the fission cross-section of uranium-235 and with increasing conviction that isotope separation was possible, in September 1941, Compton and Lawrence suggested to J. B. Conant of NDRC, who was working closely with Bush, that a third report was desirable. Since Bush and Conant had learned during the summer of 1941 that the British also felt increasingly optimistic, the committee was asked to make another study of the whole subject. For this purpose the committee was enlarged by the addition of Warren Kendall Lewis, Robert Sanderson Mulliken, and George Bogdanovich Kistiakowsky. This third report was submitted by Compton on November 6, 1941.
George Bogdanovich Kistiakowsky (1900-1982) developed the explosives RDX and HMX, was responsible for developing shaped charges and explosive lenses used in the Manhattan Project weapons, and later served as Eisenhower’s scientific advisor.
The Atomic Bomb Moves to the Top of the List
The report of May 1941 mentioned first radioactive poisons (fission products), then atomic power (for ships and submarines), and finally atomic bombs, but the emphasis was on power generation. At this moment no one anticipated atomic explosives before 1945. However a report in July 1941 on engineering suggested that the uranium program should be supported because a self-sustaining reaction was bound to have tremendous impact. Isotope separation looked to be more challenging but it should continue to receive support. A demonstration of a chain reaction was called for as a separate project from the continued acquisition of fundamental data. Lawrence considered that both reports did not go far enough on the possibility of a bomb. However additional funding was allocated to work on the chain reaction.
Finally a report in November 1941 was specifically concerned with the “possibility of an explosive fission reaction with uranium-235”. This November report said "since our last report, the progress toward separation of the isotopes of uranium has been such as to make urgent a consideration of (1) the probability of success in the attempt to produce a fission bomb, (2) the destructive effect to be expected from such a bomb, (3) the anticipated time before its development can be completed and production be underway, and (4) the preliminary estimate of the costs involved".
Bush and Lawrence met in New York City. Though he continued to support the Uranium Committee, Bush recognised that Lawrence’s assessment was not far off the mark. Bush shrewdly decided to appoint Lawrence as an advisor to Briggs, a move that quickly resulted in funding for plutonium work at Berkeley and for the mass spectrograph of Alfred Otto Carl Nier at Minnesota. Bush also asked the National Academy of Sciences to review the uranium research program. Headed by Arthur Compton of the University of Chicago and including Lawrence, this committee submitted its unanimous findings on May 17, 1941. Compton’s committee, however, failed to provide the practical-minded Bush with the evidence he needed that uranium research would pay off in the event the United States went to war in the near future. Compton’s group thought that increased uranium funding could produce radioactive material that could be dropped on an enemy by 1943, a pile that could power naval vessels in three or four years, and a bomb of enormous power at an indeterminate point, but certainly not before 1945. Compton’s report discussed bomb production only in connection with slow neutrons, a clear indication that much more scientific work remained to be done before an explosive device could be detonated.
Beginning in 1940 there was some interchange of information with the British and during the summer of 1941 Bush learned that they had been reviewing the whole subject in the period from April to July, 1941. They too had been interested in the possibility of using plutonium; in fact, a suggestion as to the advisability of investigating plutonium was contained in a letter from John Douglas Cockcroft to Ralph Howard Fowler dated December 28, 1940. Fowler, who was at that time acting as British scientific liaison officer in Washington, passed Cockcroft's letter on to Lawrence. The British never pursued the plutonium possibility, since they felt their limited manpower should concentrate on uranium-235. Chadwick, at least, was convinced that a uranium-235 bomb of great destructive power could be made, and the whole British group felt that the separation of uranium-235 by diffusion was probably feasible.
Accounts of the British opinion, including the first draft of the British report reviewing the subject, were made available to Bush and Conant informally during the summer of 1941, although the official British report of July 15, 1941, was first transmitted to Conant by G. P. Thomson on October 3, 1941. Since, however, the British review was not made available to the committee of the National Academy of Sciences, the reports by the Academy committee and the British reports constituted independent evaluations of the prospects of producing atomic bombs.
The British report, prepared by a group codenamed the MAUD Committee (Committee for Military Application Uranium Detonation) and set up in spring 1940 to study the possibility of developing a nuclear weapon, maintained that a sufficiently purified critical mass of uranium-235 could fission even with fast neutrons. The British project was called “Tube Alloys”. Building upon theoretical work on atomic bombs performed by refugee physicists Rudolf Ernst Peierls and Otto Robert Frisch in 1940 and 1941, the MAUD report estimated that a critical mass of ten kilograms would be large enough to produce an enormous explosion. A bomb this size could be loaded on existing aircraft and be ready in approximately two years. Optimistically they had concluded that a uranium bomb could be built with an explosive power of 1,800 tons of TNT.
Americans had been in touch with the MAUD Committee since fall 1940, but it was the July 1941 MAUD report that helped the American bomb effort turn the corner. Here were specific plans for producing a bomb, produced by a distinguished group of scientists with high credibility in the United States, not only with Bush and Conant but with the President. The MAUD report dismissed plutonium production, thermal diffusion, the electro-magnetic method, and the centrifuge and called for gaseous-diffusion of uranium-235 on a massive scale. The British believed that uranium research could lead to the production of a bomb in time to affect the outcome of the war. While the MAUD report provided encouragement to Americans advocating a more extensive uranium research program, it also served as a sobering reminder that fission had been discovered in Nazi Germany almost three years earlier and that since spring 1940 a large part of the Kaiser Wilhelm Institute in Berlin had been set aside for uranium research.
For example, in Germany in April 1942, Baron Manfred von Ardenne already had completed an operational magnetic isotope separator in his laboratory in Berlin Lichterfelde, and his associate Friedrich Georg “Frtiz” Houtermans had correctly calculated the critical mass of uranium-235 the previous year. The MAUD Committee calculated the critical mass of uranium-235 to be 25 pounds (about 10 kg). Robert Oppenheimer himself, before joining the Manhattan Project, had estimated the critical mass at about 100 kilograms (220 pounds). His theoretical group at Berkeley quickly increased that by three times to 300 kilograms (660 pounds). As late as August 1943, when theorists at Los Alamos provided a critical mass estimate of 40 kilograms (88 pounds), the United States' target number for enriched uranium production was still unknown.
Bush and Conant immediately went to work. After strengthening the Uranium Committee, particularly with the addition of Fermi as head of theoretical studies and Urey as head of isotope separation and heavy-water research (heavy-water was highly regarded as another neutron moderator), Bush asked yet another reconstituted National Academy of Sciences committee to evaluate the uranium program. This time he gave Compton specific instructions to address technical questions of critical mass and destructive capability, partially to verify the MAUD results.
In August 1943, the British were invited to join the Manhattan Project. This was the so-called Quebec Agreement signed by President Roosevelt and Winston Churchill. The British team included James Chadwick, Cyril Stanley Smith, Otto Frisch, Rudolf Peierls, and Emil Julius Klaus Fuchs (who later was convicted of spying for the Soviet Union).
Besides the official and semi-official conferences, there were many less formal discussions held, one of these being stimulated by Marcus Laurence Elwin Oliphant of England during his visit to the U.S. in the summer of 1941. The general conclusion was that the program should be pushed, and this conclusion in various forms was communicated to Bush by a number of people.
In the fall of 1941 Urey and Pegram were sent to England to get first-hand information on what was being done there. This was the first time that any Americans had been to England specifically in connection with the uranium problem. The report prepared by Urey and Pegram confirmed and extended the information that had been received previously.
As a result of the reports prepared by the National Academy committee, by the British, and by Urey and Pegram, and of the general urging by a number of physicists, Bush, as Director of the Office of Scientific Research and Development (of which NDRG was a part), decided that the uranium work should be pushed more aggressively.
Before the National Academy issued its third report and before Pegram and Urey visited England, Bush had taken up the whole uranium question with President Roosevelt and Vice-President Henry Agard Wallace. He summarised for them the British views, which were on the whole optimistic, and pointed out the uncertainties of the predictions. The President agreed that it was desirable to broaden the program, to provide a different organisation, to provide funds from a special source, and to open a complete interchange of information with the British. It was agreed to confine discussions of general policy to the following group: The President, Vice-President, Secretary of War, Chief of Staff, Bush, and Conant. This group was often referred to as the Top Policy Group.
The British knew that several kilograms of heavy-water a day were being produced in Norway, and that Germany had ordered considerable quantities of paraffin to be made using heavy hydrogen. It was difficult to imagine a use for these materials other than in work on the uranium problem. They feared that if the Germans got atomic bombs before the Allies did, the war might be over in a few weeks. The sense of urgency which Pegram and Urey brought back with them was of great importance.
By the time of submission of the National Academy's third report and the return of Urey and Pegram from England, the general plan of the reorganisation was beginning to emerge. The Academy's report was more conservative than the British report, as Bush pointed out in his letter of 27 November, 1941, to President Roosevelt. It was, however, sufficiently optimistic to give additional support to the plan of enlarging the work. The proposed reorganisation was announced at a meeting of the Uranium Section just before the Pearl Harbor attack (7 December, 1941).
It is odd that in some reports there is an explicit mentioned of a meeting that had already taken place on October 9, 1941, yet in other reports it is ignored or appears just as a footnote. The meeting took place between Roosevelt and Wallace and Vannevar Bush, and focussed on the atomic bomb, its potential, the cost of production, the risks including the German nuclear program, and even post-war control. The conclusion was to intensify, but not expand the program. Roosevelt understood that a great deal of money would be needed. This was not a meeting about making a bomb, but it gave Bush a free hand to find out if they could make a bomb. At the time the conclusions were limited to Roosevelt, Wallace, Bush, Conant, Henry Lewis Stimson (Secretary of War), and George Catlett Marshall (Chief of Staff).
An 'All Out' Effort is Decided
So according to the meeting of November 1941 the possibility of obtaining atomic bombs for use in the war was great enough to justify an "all out" effort, and the NDRC Uranium Section (known as the S-l Section) would be administered directly through an OSRD S-l Section, and later through an OSRD S-l Executive Committee. The membership of the reorganised S-l Section was as follows: J. B. Conant, representative of V. Bush; L. J. Briggs, chairman; G. B. Pegram, vice-chairman; A. H. Compton, program chief; E. O. Lawrence, program chief; H. C. Urey, program chief; Eger Vaughan Murphree, chairman of the separately organised Planning Board; H. T. Wensel of the National Bureau of Standards; and specialists Samuel King Allison, James Wakefield Beams, G. Breit, Edward Uhler Condon, and Henry DeWolf Smyth.
A Planning Board was set up for the technical and engineering aspects of the work, for procurement of materials and for construction of pilot plants and full-size production plants. This Planning Board consisted of E. V. Murphree (chairman), Warren K. Lewis, L. W. Chubb, George Oliver Curme, Jr., and Percival C. Keith (head of the Kellex Company). The job of this Planning Board was to have the plans ready when the time came to enter the production phase.
One aspect of the Manhattan Project that has been generally overlooked by historians was industry's contribution. Virtually overnight, the Manhattan Project created a nationwide "factory" that rivalled General Motors in size and scale despite serious wartime shortages of manpower and materials. The battle on the home front was waged without restraint. Crawford Hallock Greenewalt of Du Pont in Wilmington, Delaware, and Percival C. Keith of the M. W. Kellogg Company in Jersey City, New Jersey (which opened a subsidiary in Manhattan, NY to focus on research for the Manhattan Project), led these efforts.
Some reports go as far as to state that what made the U.S. different from those other countries that had atomic weapons programs, was not its scientific superiority, but it industrial capacity. And it was the U.S. Army that harnessed and directed that effort.
Isotope Separation
One of the big evolutions in the uranium program after June 1940 was that isotope separation became increasingly important. As information became available about the fission of uranium-235 by slow neutrons, the need to consider ways to separate uranium-235 from uranium-238 emerged as a new priority. Contracts for the development of gaseous-diffusion and gas centrifuge separation processes were recommended by the Planning Board, which would also be responsible for the heavy-water production program.
On October 21, 1941 Arthur Compton produced a status report on isotope separation. The results of the MAUD committee were reviewed, Robert Sanderson Mulliken reported on his visit to the different research teams, and Julius Robert Oppenheimer suggested that about 100 kg of uranium-235 would be needed for an effective weapon. I think this is the first time we see the mention of Oppenheimer in an official meeting. At the time he was a young physicist in Lawrence's team at Berkley. Broadly speaking Lawrence did not like the way meetings tended to focus on uncertainties, and not on the possibilities. The report issued on November 6, 1941 concluded that a "fission bomb of superlatively destructive power" was possible if they had sufficient uranium-235. 'Sufficient' was between 2 kg and 100 kg. I think for the first time they also mentioned that the bomb would be fired by bringing together parts, each less than a critical mass. So speed of assembly would be essential. Do it wrong and it would just 'fizzle'.
The consensus was that gaseous-diffusion looked feasible, but might take time to develop. The centrifuge method appeared practical and was more advanced than gaseous-diffusion. Other alternatives, such as using a mass spectrometer, were still in the laboratory phase. The time scale was three to four years, and at a cost of $50 to $100 million. The suggestion was that trial units of the centrifuge and gaseous-diffusion systems should be built and tested. The conclusion was that engineering was becoming the priority. What was not mentioned was the use of element-94 as a substitute for uranium-235. Yet Compton was of the opinion that the chain reaction would lead to a valuable power source and to the production of element-94 which could become another practical means to produce a fission bomb. This alternative was dropped in order to focus attention on a military result that could be useful in the current war. In any case the Fermi 'pile' was still not a given, so there was no way that large quantities of element-94 could be produced.
Bush sent the report of November 27, 1941 to Roosevelt with a covering letter saying he intended to form an engineering group and accelerate the physics research aimed at plant-design data.
In the re-organisation following Pearl Harbor task assignments were better defined. The Planning Board was responsible for engineering, and that meant pilot-plant experimentation and any large-scale laboratory investigations. Urey was responsible for isotope separation methods (diffusion and centrifuge) and heavy-water investigations. Lawrence was responsible for small-sample preparation, electro-magnetic separation, and 'certain cyclotron work' (meaning element-94). Compton was responsible for atomic physics, including weapon theory and chain reaction (including element-94). It was at this time that Vannevar Bush stated that when full-scale contribution started, the Army would take over.
Naturally when writing about isotope separation it is perhaps useful to first look at isotopes, and in particular the work done in 1940. Ernest Orlando Lawrence had received the 1939 Nobel Prize in Physics for the invention of the cyclotron. He was director of the Radiation Laboratory at the University of California, and in spring 1940, with Edwin Mattison McMillan and Philip Abelson, discovered element-93, and McMillan suggested the name neptunium. It was in December 1940 that Glenn Theodore Seaborg with Joseph William Kennedy with Arthur Charles Wahl finally confirmed the idea of McMillan and found evidence of element-94 (it was chemically identified in February 1941). The key was the existence of the 60-inch cyclotron. They were able to bombard uranium with deuterons and produce samples of significant size. The actual discovery was only announced after the end of WW II, but numerous scientists, including Bohr and Wheeler, had predicted the existence of transuranium elements. Initially element-94 was just seen as another component of the atomic 'pile', but it quickly became evident that if it were produced in a 'pile' and chemically separated they would have an alternative to uranium-235. Even better, they may not need to build expensive isotope-separation plants. It was Seaborg's team and Emilio Gino Segré who bombarding uranium with neutrons were able in May 1941 to confirm that element-94 would fission with slow neutrons. By the end of the month it was known that it was about 1.7 times as likely as uranium-235 to fission with slow neutrons.
It was about the same time that Lawrence decided to convert his 'old' 37-inch cyclotron into a big mass spectrometer, paving the way for the electro-magnetic isotope separation.
The scientific aspects of the work on isotope separation was kept apart from the procurement and engineering phases. The Program Chiefs H. C. Urey, E. O. Lawrence, and A. H. Compton were to have charge of the scientific aspects. Initially it was proposed that Urey should have charge of the separation of isotopes by the diffusion and the centrifuge methods and of the research work on the production of heavy-water. Lawrence was to have charge of the initial production of small samples of fissionable elements, of quantity production by electro-magnetic separation methods, and of certain experimental work relating to the properties of the plutonium nucleus. Compton was to have charge of fundamental physical studies of the chain reaction and the measurement of nuclear properties with especial reference to the explosive chain reaction. He was authorised to explore also the possibility that plutonium might be produced in useful amounts by the controlled chain reaction method.
The effect of the reorganisation was to put the direction of the projects in the hands of a small group consisting of Bush, Conant, Briggs, Compton, Urey, Lawrence, and Murphree. Theoretically, Compton, Lawrence, Urey, and Murphree were responsible only for their respective divisions of the program.
The cost of the initial work was estimated at $4-5 million, and it was stated that the Army would take over when full-scale construction was started, presumably when pilot plants were ready.
In a meeting of the reorganised S-l Section Conant called for an all-out effort given the military value of atomic bombs and that all attention must be concentrated in the direction of bomb development.
The Technologies of Isotope Separation
The detailed description of the plants built will be described on separate webpages, however it is useful here to look quickly at each of the competing technologies for isotope separation.
Isotopes differ only in the number of neutrons in the nucleus, but not in atomic number. For this reason separation of isotopes is usually based upon the mass difference and not on a chemical method. For the scientific community the centrifuge appeared to be the best option. The principles were simple, even if there were technical problems in spinning the separator fast enough. No one thought these difficulties were too serious. Purely mechanical problems were expected to yield quickly to a concerted research effort.
Gas Centrifuge
So we will start with the gas centrifuge. Today this technology is seen as small, inexpensive, and relatively simple to make. It is the device that is best known for making nuclear weapons available to the developing world. It was originally developed and tested for separating two chlorine isotopes, and through to 1944 it was one of the isotope separation technologies developed in the Manhattan Project. However the research of Harold Clayton Urey and Karl Paley Cohen at Columbia University was discontinued because it felt that it would not produce results before the end of the war. After WW II it was German prisoners of war who helped perfect the centrifuge in a Soviet labour camp. In fact the centrifuge was never a sophisticated or resource-intensive technology, and we now know that it was in fact technical feasible during the Manhattan Project, but it was not successfully developed. The project was shut down before the developers had the opportunity to solve some of the technical shortcomings.
Soviet spies quickly learned about those shortcomings and when the German team finally did solve the problems with the centrifuge it turned out to be an incredible simple device in which no precision engineering was required. It was for this reason that the Chinese started a centrifuge program as early as 1957 (a program that took decades for the U.S. to detect). By 1975 India, Brazil and Pakistan all had centrifuge programs, and Pakistan passed on the technology to North Korea in the mid-1990's. Today experts have estimated that at least 20 countries have built or acquired centrifuge technology.
The existence of isotopes were first announced in 1918 by Frederick Soddy and John Arnold Cranston, and it was this work that inspired H.G. Wells to write about atomic bombs being dropped from biplanes in The World Set Free. It quickly became evident that the only way to separate chemically identical atoms of the same element was to exploit their minute difference in masses and their radioactive properties. It was Frederick Alexander Lindemann and Francis William Aston (the inventor of the mass spectrometer) who wrote at the time that the centrifuge was the most promising method for isotopes separation. However throughout the 1920's the engineering problems proved formidable. The simple problem was that no one could get the centrifuge to spin fast enough. Finally when they could spin it fast enough the air friction heated the centrifuge so much that it cancelled the separative effect. It was Jesse Wakefield Beams in 1934 who had the insight to build a centrifuge inside a vacuum chamber, and he managed to separate chlorine-35 from chlorine-37.
In fact the early centrifuges were based on a shaft-mounted rotor, and were known from early times but it was Karl Gustaf Patrik de Laval in 1883 who introduced the flexible shaft that was able to shift under the force. It was Theodor Svedberg, a colloid chemist, who in the 1920's invented the first ultracentrifuge, machines that work at 1,000's rps. His centrifuges were housed in a low-pressure hydrogen atmosphere to reduce friction and equalise temperature differences. His research earned him the 1926 Nobel Prize in Chemistry for providing evidence of the physical existence of molecules. Émile Henriot investigated ways to spin tops at extremely high angular velocities using air-jets, and the technique was used to construct the shaft-less ultra-centrifuge (Henriot was also the first person to show that potassium and rubidium are naturally radioactive). Examples of these shaft-less centrifuges could spin at 4,000 rps, however the next limitation was friction with the atmosphere. Beams placed his rotor in a high vacuum. His first centrifuge used a flexible shaft attached to an air-drive turbine and suspended in a vacuum. As with earlier systems this design with a flexible shaft allowed the rotor to spin about its own inertial axis. The next step was to try to use magnetic fields to support the rotor. Beams design used an external electro-magnet to lift the rotor and let it spin freely and rotate about its own inertial axis. The problem with this design was that the rotor could move up and down if the balance between the magnetic field and gravitational pull was not maintained. Beams used a beam of light and a photoelectric cell to obtain positional information and feed that to the current powering the electro-magnet. This design could spin at 1 million rps. One of Beams students would go on to design a field-supported centrifuge that would be commercialised as a sample preparation tool found in almost every laboratory (this ultracentrifuge was marketed by the industry leader Spinco). Beckman bought Spinco in 1955, and the ultracentrifuge was used to prove James Watson and Francis Crick's theory on DNA replication. Through into the 1970's the analytical ultracentrifuge was one of the most conspicuous and valuable tools in microbiology and biochemistry laboratories. There is a world of different between a very small object spinning at 1 million rps for short periods of time, and a laboratory ultracentrifuge from the 1950's that was the size of a small car and could manage up to 100,000 rpm.
Already from 1940 the centrifuge was the preferred method for the large-scale enrichment of uranium. Beams and Alfred Otto Carl Nier had separated small uranium samples in a mass spectrometer and neither felt that the technique was scalable. On the other hand Beams was convinced that the centrifuge was potentially scalable for uranium enrichment. From 1941 the largest share of funding went to Beams and the centrifuge, four times more than that allocated for gaseous-diffusion. Initially a small centrifuge was designed at Columbia University and built by Westinghouse Research Laboratories, and a 'high-performance model' was developed by Beams at the University of Virginia. These machines were based upon the laboratory apparatus that was used to separate chlorine isotopes. The approach of Beams was to use the funding to test a primitive single-batch centrifuge concept called the 'evaporative centrifuge' on uranium hexafluoride (UF6). Most experts now consider this as a poor use of wartime funding. In fact the engineering development had already started with Westinghouse before the first meeting of the Planning Board in July 1941.
We start with a binary gas mixture of two molecules, one slightly lighter than the other. In very simple terms the gas enters the centrifuge and undergoes a centripetal acceleration. The rotor is turning at a constant speed (constant tangential speed), but the gas molecules will feel an acceleration (change of direction of the velocity) as they move in the uniform circular motion, and that acceleration is in the direction of the change of velocity, which is pointing towards the centre of rotation. But the molecules feel an apparent force, equal and opposite to the centripetal force (the centrifugal force), drawing them away from the centre of rotation, and this is caused by the inertia of the molecules. So the denser or heavier molecules move towards the exterior wall of the rotor, and the lighter molecules don't move as much and remain closer to the centre. The lighter fraction is richer in uranium-235 as compared to the heavier gas fraction which has been depleted of uranium-235 and is thus heavier with uranium-238. In any one centrifuge the different is small, so in order to be effective several centrifuges are connected in series (a cascade).
One of the key features is the way the centrifuge changes the direction of enrichment from radial to axial, by inducing a weak axial circulation of the gas, called a counter-current flow. It is this 'trick' that allows the centrifuge to remove enriched and depleted gases at each end. What it creates are two streams going in opposite directions from one end to the other. The stream nearer the axis is becoming enriched, whereas the stream near the outer wall is becoming depleted. In this sense the centrifuge acts as a kind of distillation column. The reason for creating a counter-current flow is the axial separation factor is much larger that the radial separation factor. This counter-current can be created by heating the bottom end and cooling the top end. The other method is mechanical, where a stationary object (disk) is placed in the gas stream near the bottom end. The scoops can act as mechanical obstacles as well, creating a drag which changes the tangential velocity of the gas and at the same time warms the gas. These two effects can also create the counter-current.
The overall performance of a centrifuge is related to the shape of the flow profile and the strength of the counter-current. In theory the performance has a 4th-power dependence on the peripheral speed of the rotor and is directly proportional to the length. We can see from this the importance of the rotor speed.
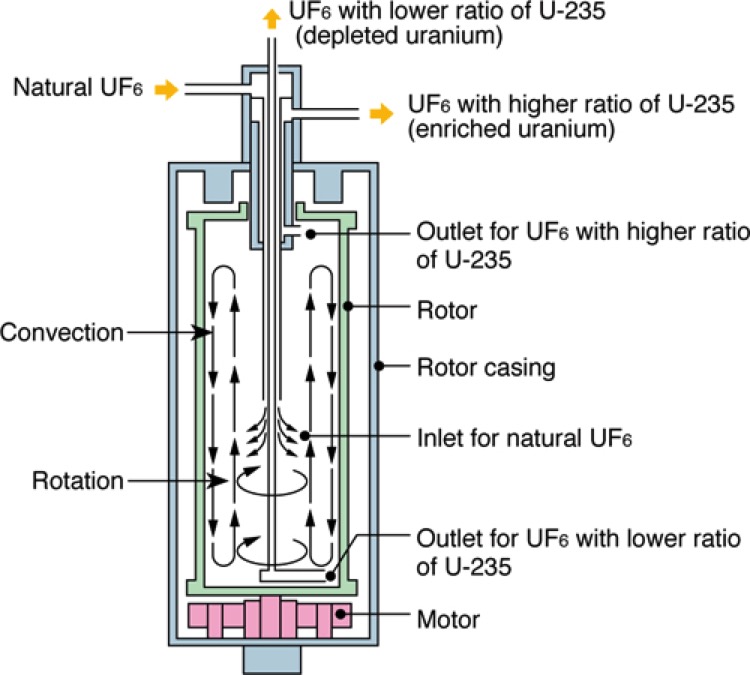
What we can see above is the so-called short bowl centrifuge developed after the WW II (see below for further details). We can see some of the basic features. We can see the rotor in green, which spins around its axis sitting on a thin, flexible steel needle bearing (today the speed is about 22,000 rpm or about 350 m/s for smaller centrifuge units). The idea of the needle bearing is that it is not a rigid bearing but it allows the rotor to pivot like a top. The needle is sitting in a depression in a hard metallic plate, which in turn is centred elastically and is damped by oil friction. The upper bearing of the rotor consists of a hollow cylindrical permanent magnet mounted in a damped gimbal through which a steel tube passes without touching the wall. Three tubes for the insertion and extraction of the gas pass through this opening. You have a feed tube which ends in the middle of the rotor. The other two exit tubes actually terminate at opposite ends of the rotor (not easy to see in the diagram above but they are marked 'outlet'). These tubes have their ends bent into a hook-shape curve ('scoops') perpendicular to the axis of rotation. These scoops make use of the high impact pressure in their vicinity to transport (extract) the heavier and lighter gas fractions. The extractor tube for the enriched uranium hexafluoride has a slightly reduced angular velocity (as compared to the other tube) which induces the counter-current flow. The extraction tube for depleted uranium hexafluoride is in fact protected by a lower plate or baffle which is not shown in this diagram. This baffle has holes in it to allow the gas to leak from the main rotor cavity into the area near the scoop. The baffle is needed at one end to stop the scoop imposing a vertical flow that would counteract the circulatory flow generated by the scoop at the other end. The vacuum cylinder or stator (the blue part near the top nozzle) has screw type groves in the wall, and combined with the rotating tube act as a molecular pump to maintain a vacuum around the end of rotor, reducing gas friction, and thus any heating. We can see the electric motor where the armature is a flat hardened steel plate attached rigidly to the bottom of the rotor. The field winding (which is marked motor) is located in the vacuum and is fed from outside by an alternator at a frequency synchronous with the speed of the rotor.
Wikipedia has an interesting article about both Gernot Zippe and Manfred von Ardenne. Zippe had worked on isotope separation for the German nuclear weapons program. At the end of the war he was kidnapped and imprisoned in the Soviet Union. He was forced to work on a centrifuge project in a physics institute near Sukhumi, a small seaside resort town in Georgia (the institutes were often called sanatoriums). The institute was run by Manfred von Ardenne who after WW II agreed to work for the Soviet Union on isotope separation as part of the Soviet atomic bomb project. Although the centrifuge option was abandoned during the Manhattan Project the Soviet Union continued to develop the technology. Through 1947 the objective was to study mechanical and metallurgical problems associated with the rotor speed. They were able to attain about 80,000 revolutions/minute (rpm), but the target was 150,000 rpm. The next step was using a 'special aluminium alloy' for a reinforced rotor 40 cm long. A considerable number of tests were made to eliminate vibration and any unbalanced movement. Sleeve and needle roller bearings were used to reduce friction, and all the bearings were cooled in chambers of liquid air. In particular a needle bearing allowed the centrifuge rotor to spin on a needle point, with very little friction. This type of 'point' bearing was an adaption of the jewel bearing used in watchmaking and in some electric motors dating from the 1900. The other type of bearing were made a bit more flexible and with weak damping, allowing the centrifuge to adjust itself so that it could spin on its axis without vibration. Using tight bearings and strong damping often forced the centrifuge to spin off-axis and vibrate. This idea was an implementation of Karl Gustaf Patrik de Laval's principle of self-balancing, used for steam turbines since 1889. In addition the internal walls of the rotor were lined with permanent magnets, and the rotor itself was driven asynchronously by a high frequency transmitted via a continuous winding wrapped around the rotor chamber. The idea of an indirect drive came from an indiction-welding system build by another German POW working in the same institute. With all these innovation they finally attained 150,000 rpm. The key appeared to be proper cooling using both air and water. An efficiency claim for just one unit was 2% (see design below). Zippe has a specific type of centrifuge named after him, the Zippe-type centrifuge. He finally was released in 1956 and for a period worked with Beams at the University of Virginia, before retiring back in Europe. The work Zippe did in the University of Virginia concerned what he called the short-bowl centrifuge, and the reports are in the public domain (the photograph above is the prototype built at that time).
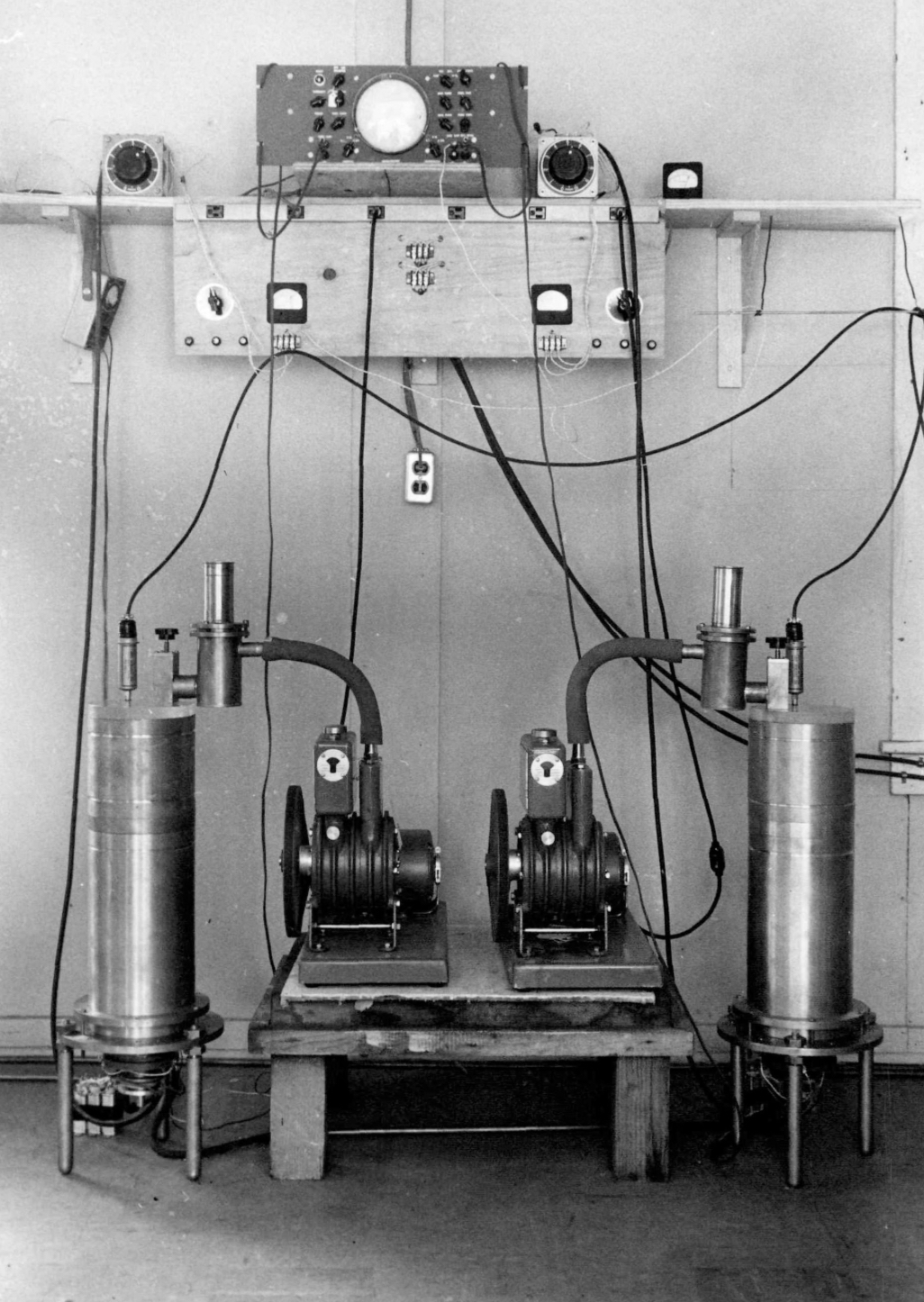
The experts who have looked back over the development of the centrifuge in Russia noted that already in 1940-41 they too were interested in thermal diffusion and the gas centrifuge. They too felt that the thermal diffusion had the greater initial research base, but many felt that it would consume more power than could be extracted from the 'purified' uranium-235. The only centrifuge program at the time was run by a German émigré called Fritz Lange. His centrifuges were heavy, noisy, and clumsy machines that operated horizontally on roller bearings and produced no measurable enrichment. The Russians at the time thought that the U.S. was building a nuclear bomb, so they also began to explore that possibility. Georgy Nikolayevich Flyorov also worked out that a fast-fission chain reaction was necessary and that enriched uranium would be required. It would appear that the Russians were also aware of the MAUD report, so the laboratory of Igor Vasilyevich Kurchatov also took on the work of Lange on centrifuges. The initial design of Lange was about 60 cm long and 25 cm in diameter. Internally the rotor was divided into 60 chambers, and the total weighed around 2½ ton and consumed 3 kW. The key to its failure was that the rotor's maximum speed was only 130 m/s, well below the necessary speed to effect measurable separation. Lange tried to improve on the design by making the centrifuge longer, but what happened is that the 5 m long rotor sagged in the middle and caused destructive vibrations (and at high speeds they could snap in the middle). The bearings on the Lange centrifuge only lasted eight to ten hours, and not the 99 days with Beams's centrifuge. In 1944 Kurchatov learned that the U.S. had chosen diffusion over centrifuges, and he was receiving information from the spy Klaus Fuchs who was working on diffusion membranes. It was at this time that Lange was transferred away from the laboratory as it turned to gaseous-diffusion. This was probably a mistake because the centrifuge ultimately proved the more efficient, however a horizontal centrifuge would never have worked (Beams had published some 6 years earlier that they were using vertical centrifuges). It was the German Max Christian Theodor Steenbeck who offered to work for the Russian on centrifuges and he started with copying the work of Beams. Gernot Zippe worked in that team.
In 1949 the Russian discovered, like the Americans, that with gaseous-diffusion they could not get past 40% enrichment. This gave Steenbeck the chance to propose to use centrifuges for a top-up plant to go from 40% to 90%, but it was not certain that the design was ready for mass production. The centrifuges were expensive to build and operate. At the time they relied on external vacuum pumps to maintain the vacuum seal around the rotor, and in addition they needed compressors to pump the uranium gas from one centrifuge to another. An industrial application would consume enormous amounts of electricity, as did gaseous-diffusion plants. Steenbeck proposed, as did Beams, to build 5 m long centrifuges, reducing the number of vacuum pumps and compressors needed. A new problem occurred, the long rotor was not rigid enough and vibrated. The solution was to use shorter rotors interconnected by bellows to compensate for the vibrations. However, a certain Voskoboynik solved the problem with gaseous-diffusion plant, so the top-up option was never needed. Work continued to 1952 where the entire operation was transferred to Leningrad and integrated into the Soviet nuclear production program. It was there that the engineers added a pitot tube to extract the gas from the centrifuge. The tube harnesses the rotational momentum of the gas to pump it from one machine to the next (an idea that had been used in centrifuges since 1901 to eliminate compressors). They also added a spiral-grooved sleeve around the top of the rotor so the outside of the spinning centrifuge tube acts as a self-evacuating vacuum pump, and idea called a Holweck molecular pump invented in 1922. All the improvements meant that there was no need to move to long centrifuges, and they focussed on building short, simple centrifuges. A plant came online in 1957 and had 2,435 centrifuges by 1958. Intelligence estimates put the production of such a plant at one highly-enriched implosion device every two years. According to Zippe's estimates 20,000 centrifuges of this type could produce about 1 kg of 96% enriched uranium per day. This assumes 100% efficiency, but in practice 20% efficiency was a practical target.
Today the reality is that the gas centrifuge is the most economically efficient way to enrich uranium for peaceful power-reactor fuel, but a so-called 'rapid-breakout' would also enable a country, using the same technology, to produce nuclear weapons before there is time for a political response. In addition clandestine plants are difficult to detect as compared to nuclear reactors or large gaseous-diffusion plants. Centrifuge plants use little electricity and produce little detectable signal, so the plants are much easier to hide. Check out this article for an extended review of centrifuge technology.
At a meeting on November 10, 1942 it was decided to pursue a full-scale gaseous-diffusion plant, with the electro-magnetic method held in reserve, and to drop the centrifuge. These decisions were taken after an extensive review that showed that, despite initial high expectations, the centrifuge had not met those expectations. Many reports on the Manhattan Project don't mention the centrifuge after that date, however other reports indicate that the centrifuge enjoyed a brief revival a few months later.
Gaseous-Diffusion
After nuclear fission was first discovered, the next step was the determination that the fission reaction was due to the isotope uranium-235. Once the idea that a self-sustaining chain reaction might be possible, the problem became one of trying to concentrate uranium-235 relative to the far more abundant uranium-238. As mentioned elsewhere the task was to use the difference in atomic masses between the two isotopes, and the difficulty was that the difference is very small.
The 'classical' method to separate two gases is gaseous-diffusion, based upon the experimental finding of Thomas Graham in 1829 who pioneered the study of the diffusion of gases. He found that the rate of effusion of gases through small apertures was inversely proportional to their molecular weights. Francis William Aston had used the principle of Graham to partially separate the neon isotopes. Later William Draper Harkins would apply the method to chlorine, and Gustav Ludwig Hertz achieved almost complete separation of neon isotopes, by recycling the gas through many stages.
The basic physical underlying the gaseous diffusion method is the so-called 'equipartition principle' of statistical mechanics. The basis is that in a gas consisting of several types of molecules each type will have the same average energy of motion (kinetic energy). This equality of average energies is attained and preserved by the enormous number of collisions between the molecules in the gas. These collisions ensure that any excess energy will be rapidly shared equally with all the molecules, this is the meaning of 'thermal equilibrium'. The kinetic energy of a molecule is related to its mass and it's velocity, therefore molecules having the sane kinetic energy will have average velocities which differ in inverse proportion to the square roots of their masses. If we take a uranium hexafluoride molecule with uranium-235 and another with uranium-238 the ratio of their velocities is 1.0043 with the lighter molecule moving very slightly faster 'on average'. The idea of gaseous-diffusion is to exploit this small different in average velocities by forcing the gas mixture to diffuse through a porous barrier under a pressure difference. The faster molecules will encounter the holes more often than the slower ones, and therefore be slightly more likely to pass through the barrier. The gas that emerges on the other side of the barrier will be slightly 'enriched' with the lighter isotope. The ideal of 1.0043 is never met since it's dependent upon the concentration of the desired isotope on the 'feed' side which will gradually decrease as the enriched mixture diffuses through the barrier (a value of 1.0014 would be good practice).
A uranium hexafluoride molecule has a diameter of about 0.7 nm and at a pressure of about ½ atmosphere and at a temperature of about 80°C the average separation between molecules will be about 5 nm. The mean free path under these conditions is about 85 nm, so the average pore size in the barrier must be somewhat less, say 25 nm. To get this pore size you could imagine packing spheres together of about 100 nm, which would leave gaps of about 25 nm. So one could imagine a barrier made of sintered nickel power, i.e. packed under high pressure and at a high temperature. And naturally this barrier must be very thin (few micro-meters) yet withstand a pressure differential of 0.2 to 0.5 kg/cm2. In most cases the barrier would be prepared as a tube made of the thin porous layer deposited on top of a structural layer allowing the barrier to work under higher pressures.
It was on May 21, 1940 that George Bogdanovich Kistiakowsky suggested gaseous-diffusion as a possible way to separate uranium isotopes. At the time he was thinking of the work of a certain Charles G. Maier in developing ways to separate mixed gases. Later he found that the approach of Maier had some important defects, but that the technique looked appealing. It was in early July 1940 that Urey suggested a barrier made of special glass, and initial reports by Kistiakowsky on October 14, 1940 looked promising. He would bow out of the topic due to work pressure, but Urey, Dunning, and others in Columbia University continued through to the spring 1941 to look at alternative barrier materials. The problems were considerable. The barrier needed to have billions of holes with a diameter of about 1/10,000th of a millimetre. Yet the barrier needed to withstand a considerable pressure difference and mechanical strain, not to mention the highly corrosive nature of uranium hexafluoride. And the process must not leak to the air, otherwise uranium oxyfluoride would be formed and clog the barrier. And industrial plant would need 10,000's of square meters of the barrier and 1,000's of stages, and would need to work as a process (not by batch). Yet to the experts the technical difficulties appeared less than with the centrifuge.
There are several different separation processes, the liquid thermal diffusion process being the most significant. Thermal diffusion processes using gases also appeared attractive, however after some test experiments the process appeared impractical for large-scale separation. One lone voice, Philip Hauge Abelson, argued for liquid thermal diffusion, and he received funding to continue that work at the Naval Research Laboratory.
Input from the British (MAUD Committee) suggested that isotope separation using the gaseous-diffusion method offered the best chance of success.
Diffusion and effusion are two slightly different concepts, diffusion is the net movement of atoms or molecules from one region of higher concentration to a region of lower concentration. It is driven by the gradient in chemical potential of the diffusing species, i.e. the energy adsorbed or released due to a change of particle numbers of the species, it's what also drives chemical reactions and phase transitions. Effusion is the process in which a gas escapes from a container through a hole of diameter considerably smaller that the mean free path of the atoms or molecules. Typically this is a gas leaking through a pinhole, where the leak is driven by a pressure difference between the inside of the container and the exterior.
The approach was to look for a volatile species of uranium, i.e. uranium hexafluoride, as a working substance. The technique consists of a series or 'cascade' of diffusion stages. The 'converter' in each stage consists of a cooler and a 'diffuser'. The diffuser is a tank-like piece of equipment which enclosed a 'barrier'. The barrier is a thin, porous, metallic membrane containing billions of submicroscopic holes.
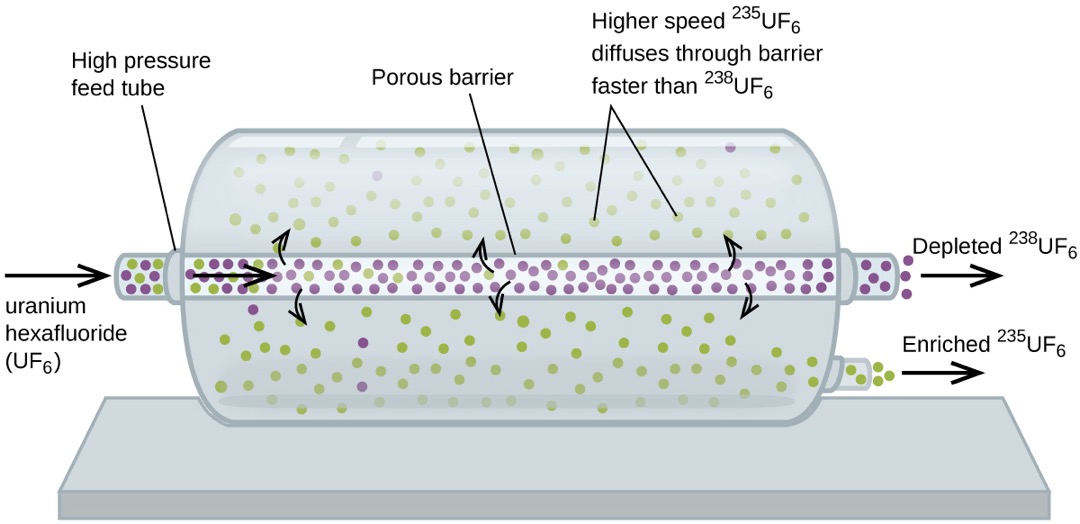
The idea of a cascade is that a pump supplies a converter with a continuous stream of un-diffused gas from the stage above, mixed with diffused gas from the stage below. The cooler is there to remove the heat from the pump and to maintain a constant operating temperature (the cooler removes about 95% of the energy added by the compressor). The idea is that half of the gas fed by the compressor to the converter diffuses through a barrier, becoming slightly 'enriched' with the lighter component in accordance with Graham's Law (i.e. the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles). What this means is that the molecules with a lower molecular weight (uranium-235) have a higher molecular velocity and diffuse more readily through the barrier pores. Consequently, the fraction of the gas that passes through is slightly enriched in uranium-235 and the gas that does not pass though is slightly depleted in uranium-235. The theoretical enrichment of one converter is about 1.0043. The 'concentrated' or enriched gas is then pumped to a next higher stage. The remaining half of the gas leaves the converter and is pumped to a lower stage. Naturally the product is withdrawn from the top of the cascade, and the waste or depleted gas is removed from the bottom of the cascade.
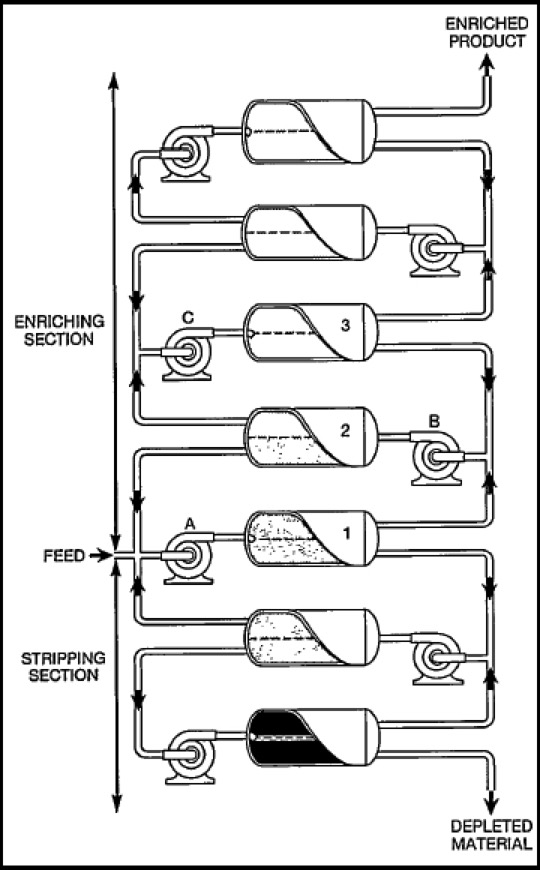
Above we see a schematic of the cascade process, and below a slightly more detailed view of the way converters are linked together in the cascade.
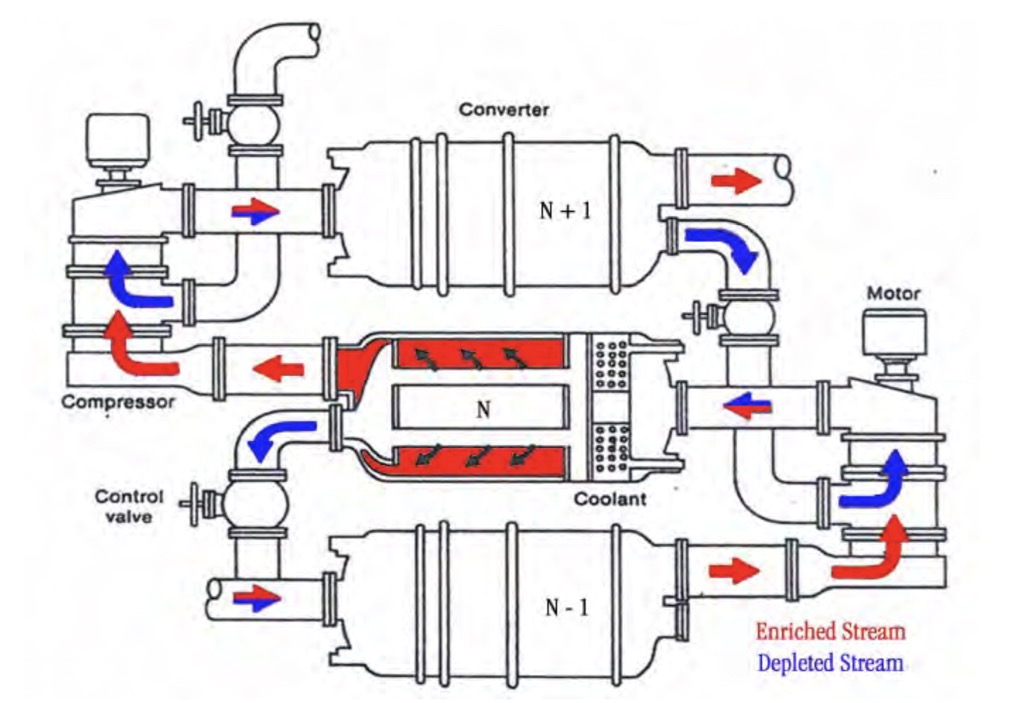
The converter with its associated pumps and coolant, etc. is called a 'stage', and today one of these stages is about 4 m in diameter and about 7.3 m long, and contains tightly packed diffusion barrier tubes. Below we can see one stage (note the 'hard-hat' in the lower left corner). This is a large converter in the low enrichment section of the cascades, smaller converters are found in the higher enrichment sections.
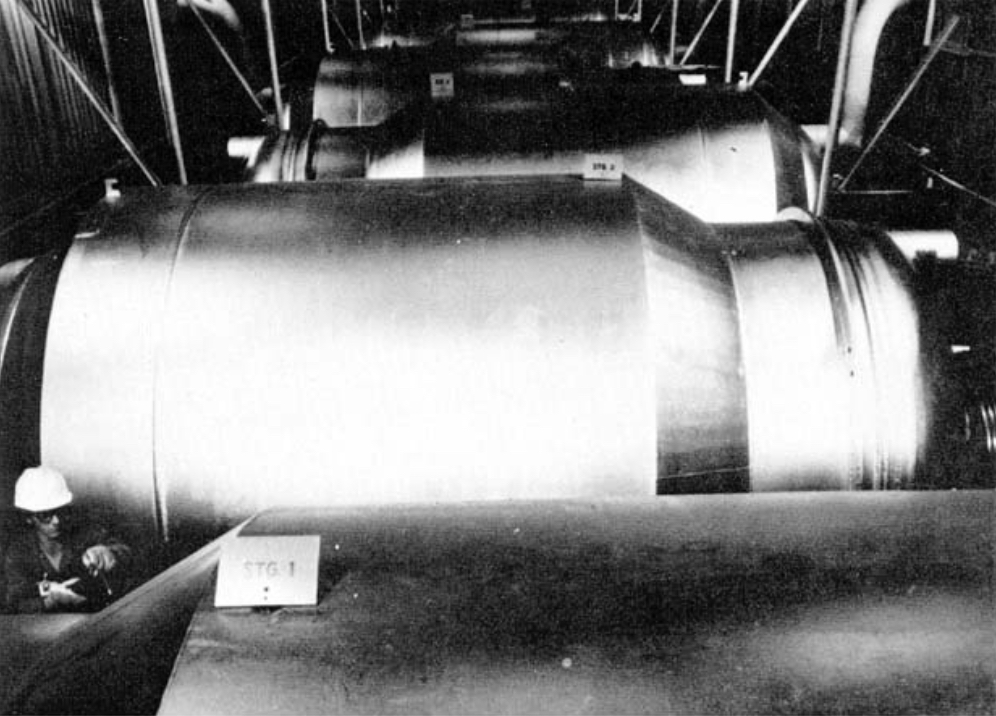
Several converters are grouped together in a cell, as seen below. A large facility will have 1,000's of these converters. A large-scale plant could have 5 different sizes of converters, so as to optimise energy consumption, which is in any case significant. Above we see the largest converter which is attached to a 3,300 horsepower motor, whereas the smallest converter might be attached to a motor of only 15 horsepower.
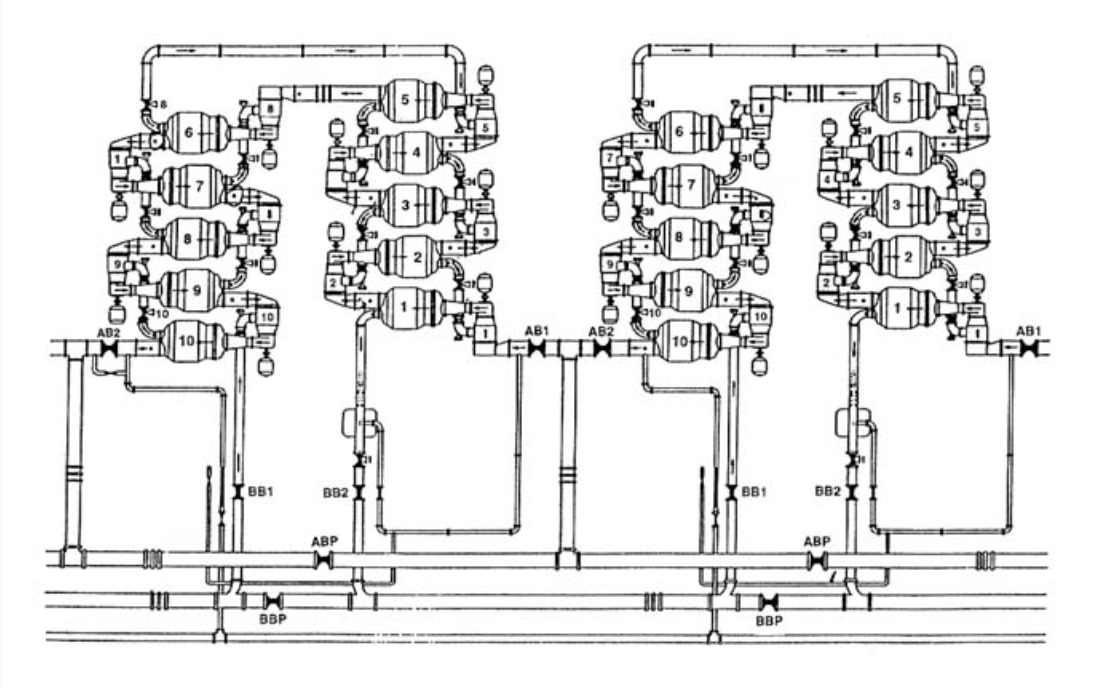
If we look more closely at the barrier, in order for a maximum separation the porous barrier material must meet exacting standards for 'diffusive' flow to occur. The pore sizes must be such that individual molecules collide only with the pore walls rather than with each other (see below). In our case to ensure diffusive flow, a uniform pore size of less than two-millionths of an inch diameter must be obtained. The hexafluoride molecule of uranium-235 has slightly less mass than the hexafluoride molecule of uranium-238, thus it can pass through a properly constructed barrier more quickly.
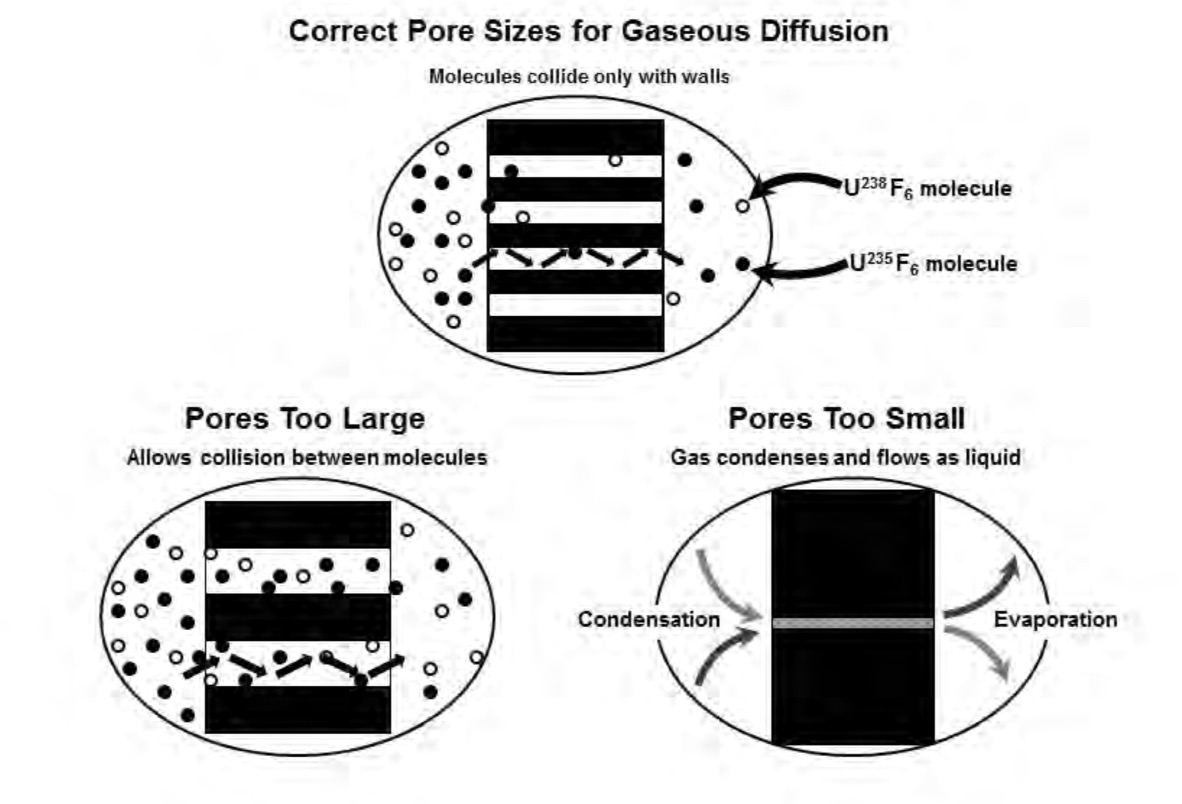
Since the process itself is so simple, there must be some serious technical difficulties. These start with the fact that uranium hexafluoride is extremely reactive with water, it is also very corrosive to most metals, and it is not compatible with organics such as lubricating oils. These system are almost entirely built out of nickel-plated steel, Monel (nickel alloys), and aluminium. The environment in which the uranium hexafluoride is found makes it essential that the barrier retains its separative capability over a long period of time, i.e. the last thing you need is to constantly intervene in such an aggressive environment. Another problem is that because the natural enrichment is very low in uranium-235, the feed will be very large quantities of hexafluoride gas which needs to be pumped through the initial converters.
In 1940 and 1941 the investigation of gaseous-diffusion was limited to testing naturally porous substances, various metal alloys and some metal powers. In addition some tests were performed on the corrosion resistance of some materials, but the laboratory in Columbia University did not even have a pump that could operative with corrosive gases.
When it was decided to build a pilot plant, the first step turned out to be the need to develop components that could withstand the extreme operating conditions. For example there was a need for high-speed pumps that could operate with corrosive gases for extended periods of time without leaks. Another problem was seals and lubricants, and of course there was the ever present problem of the barrier. And not just a barrier, but one that could be produced in 10,000's of square metres.
Finding the right barrier was the most persistent problem. Francis Goddard Slack tested hundreds of materials, unfortunately certain materials that might work well in the laboratory could not be used in a large-scale production plant. Not only did the material have to undergo tests as barriers, but they also had to be tested for resistance to corrosion. Even the best barriers found during the summer of 1941 were simply (a) not sufficiently corrosion resistant, (b) too brittle and fragile, and (c) were not sufficiently uniform. Attention turned to nickel barriers, which were at least resistant to corrosion.
Lacking information on the barrier, it was difficult to design the converter to be used in the large-scale plant. But next to the barrier the pumps were the biggest problem. The specification was simple, the pumps needed to pump a relatively heavy, highly corrosive gas at high velocities, with no leakage into or out of the system. By summer 1942 they were able to test some different type of pumps with seals etc., but no one was able to produce anything more than a test-tube of uranium-235.
Reading through the reports one is struck by the continued search for a decent barrier by a team of 80-odd people at Columbia University, alongside the work of the Army (with Kellex and Union Carbide) who were trying to specify, design and build a large-scale production plant at Oak Ridge.
Advances were made, they designed and successfully tested a new seal on the pumps, but the barrier remained elusive. After a year of intense research and testing they still had not found a good barrier. By then they would have accepted a 'bad' barrier, i.e. one that had stable (but bad) separative qualities, but worked over long periods of continuous operation. How to make sub-microscopic holes that did not clog or plug? How to make a fine barrier that was still mechanically strong? By the end of 1942 everyone thought that such a barrier did not exist. The nickel barrier had shown some early promise, but was too brittle and fragile. A barrier made of nickel powder provide to have poor separative qualities, but appeared the more likely to be successful. The experts thought there were so many potential variations and combinations that they ought to find one that worked. During experiments in 1942 they had seen that even a minor change altered considerably the separative qualities of the barriers. However with so many options they tested hundreds of different barriers, but always felt that there were more that could be tested. At the end of 1942 the 'Norris-Adler' type family of barriers looked the most promising.
The approach with gaseous-diffusion has been likened to a cook-book. Was the difference between a good and bad barrier just a question of person touch? Being empirical perhaps it would all depend on some intuitive innovation, Did one sample represent a type of barrier as a whole? If they found a good barrier could they work out how to reproduce it? If they found a good barrier, could they make a lot of it?
There was progress, the 'Norris-Adler' barrier had been limited to laboratory trials in 1942, but in January 1943 it was decided to try and develop a continuous production process for the barrier, and in July it was operational in the laboratory in Columbia University. By that time the construction of the full-scale barrier production plant had also been started, and the company contracted to do the work (Houdaille-Hershey) also built its own pilot plant. In mid-1934 the results were not totally negative, and the plant started to produce barriers for testing. The material was susceptible to corrosion and plugging, but the biggest problem was the lack of uniformity, and in particular the separative quality. Barriers from the same batch would be totally different, so good, others not. The approach of Columbia was just continue to test samples and hope.
Building and testing gaseous-diffusion test units continued during 1943, but the idea to build a pilot plant had been abandoned, even if the building of a 10-stage unit was not immediately stopped, it just languished. The best they could manage were converters about 10 cm in diameter, and in the winter of 1943 they managed to work steadily on testing small barrier samples.
Electro-Magnetic Separation
Between 1927 and 1931 Ernest Lawrence invented the cyclotron. He had certainly read about the concept of the linear accelerator put forward by Gustaf Ising in 1924, and its first realisation by Rolf Widerøe in 1928. Lawrence was interested in using the idea to study the interaction between protons and the nucleus. Theory dictated that he would need 1 MeV protons to do that, but that would have required a prohibitively long tube. Like many great ideas, it is all about transposing one idea into another context. Lawrence realised that he could use a magnetic field to confine charged particles to a circular trajectory. The trajectory in a given magnetic field would depend upon the particles velocity. He managed to build a way to apply successive resonant accelerations through the application of a radio-frequency voltage across the gaps of magnetic pole pieces, the so-called 'dees'. In 1929 he managed to generate a 1.3 keV proton, then it was 80 keV, and in 1931 he had his 1 MeV protons.
By 1941 the cyclotron of Lawrence had become a powerful tool for nuclear physicists, but now he had a new idea. He decided to turn his old 37-inch cyclotron into a mass spectrograph. A cyclotron accelerates charged particles from a central source along a spiral path created by a static magnetic field (the 'dees'). The particles are accelerated along that path by a varying radio-frequency electric field. Below we can see the basic components. In the photograph we can see the 'dees' marked with D, and 1, 2, and 3 are radial probes that can be inserted into the charged particle stream.
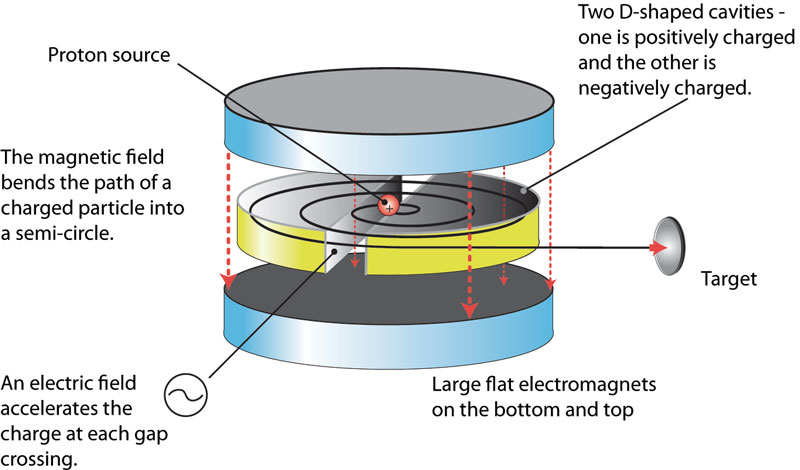
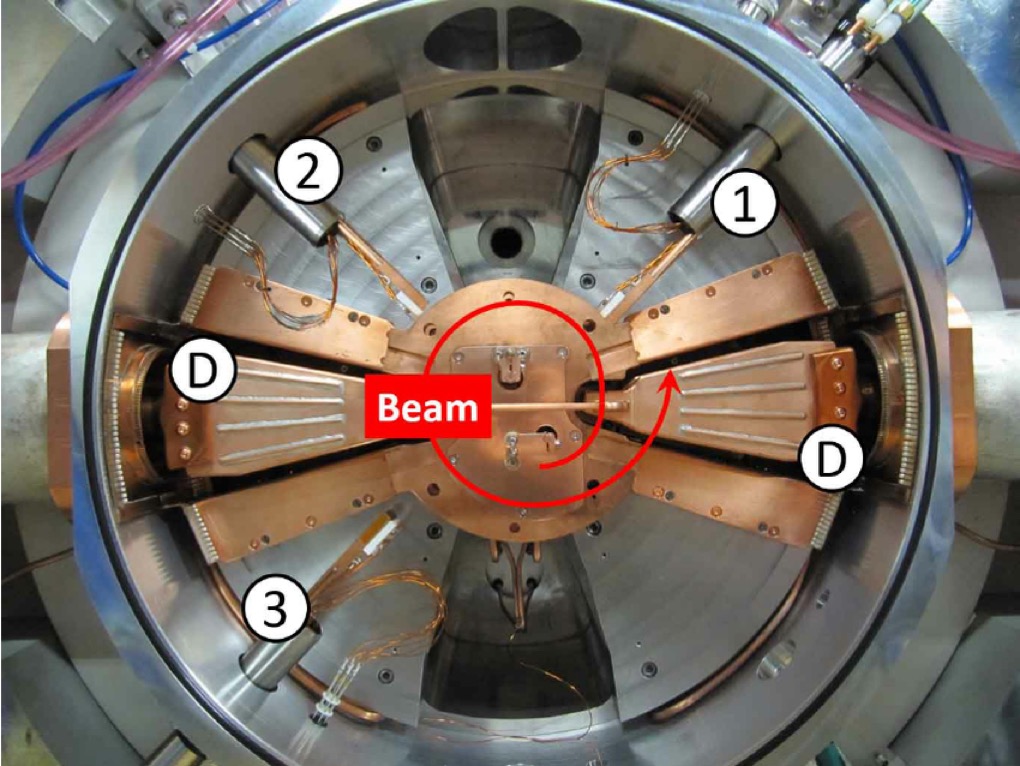
A mass spectrometer is a device that also has a ion source (e.g. one that creates ions by first vaporising and then ionising a sample), an accelerator section, an 'analyser' or deflector consisting of electric and magnetic fields that alters the ions trajectories dependent upon their relative mass-to-charge ratio, and one or more collectors or detectors.
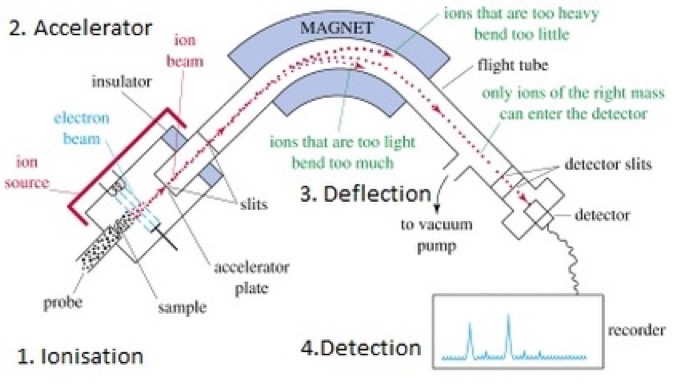
The mass spectrometer and the cyclotron has some striking similarities. Both required a high-vacuum chamber and a large electro-magnet. Lawrence knew that Nier in Minneapolis could not produce enough uranium-235 samples, so his idea was to try to obtain larger samples of uranium-235. It is true that the mass spectrometer of Nier did separate uranium-235 from uranium-238 but the quantities were extremely small (it would have taken 27,000 years for his single mass spectrometer to separate one gram of uranium-235).
The idea of electro-magnetic separation must have been in the back of Lawrence's mind, but he also knew that there was a problem. It was generally believed that with a larger beam of positively charged ions, they would start to repel each other in the same way electrical charges do (this is called the space-charge limitation). A good example is if you imagine a current flowing through a vacuum between a cathode and an anode. That current can't exceed a certain maximum value because of the modification of the electric field near the cathode. In simple terms the positive ions don't see the negative cathode because the other positive ions in front of them are shielding it. So the assumption was that if there were too many positive ions they would repel each other and the scattering would disrupt the separating effect. All the texts I've read state that from his experience with the cyclotron Lawrence thought that the air molecules in the vacuum chamber would have a neutralising effect, i.e. negatively charged particles in the air kept the beam from dispersing under its own influence.
Starting in late November 1941 Lawrence replaced the cylindrical vacuum tank that occupied the 8-inch gap between the magnet poles of the cyclotron with the components of a mass spectrometer (similar to the one used by Nier). In the first part of the source electrical heaters vaporised solid uranium chloride. In the second part of the source electrons from a heated cathode ionised the gas. A slit 2-inches long and 0.04-inches wide permitted the positive charged ions to escape into the vacuum tank. In a plane perpendicular to the magnetic field, the beam followed a 180° circular path about 60 cm in diameter to a detector on the opposite side of the vacuum tank. The lighter uranium-235 ions would be bent just a little more, and a moveable shield with a very narrow slit enabled the operator to select which part of the beam should be collected. The tests worked well and they managed to demonstrate an enrichment to about 3%, but the quantities were of course micro-grams. The key message was that even with this device the beam current was about 5 micro-amperes, or 10 times more than that Nier's mass spectrometer. And the way it operated showed that the space charge did not affect the beam. Within a month they had increased the beam current to 1.4 milli-amperes, and had produced three 75 micro-gram samples of 30% enriched uranium.
The objective of this device was different from a mass spectrometer. In an ionised gas containing two different isotopes, in any given time the lighter ions will travel a greater distance than the heavier ions. High frequency electrodes placed along the ion path could be used to trap one isotope and let the other continue to the target (e.g. a kind of linear chopper). This way there was no need to focus the beam on a slit, and all the ions could move in all directions. And there would be no need to bend the ions in a precise circular path. In fact there were a number of different ways to separate and collect ions from different isotopes, and the bigger the weight difference, the better they worked. The problem was to develop a technology to produce large quantities of uranium-235 quickly.
By February 1942 Lawrence had already planned a better model exploiting the 37-inch magnet. He replaced the cyclotron tank with a C-shaped vacuum chamber (rather than a large flat disk).
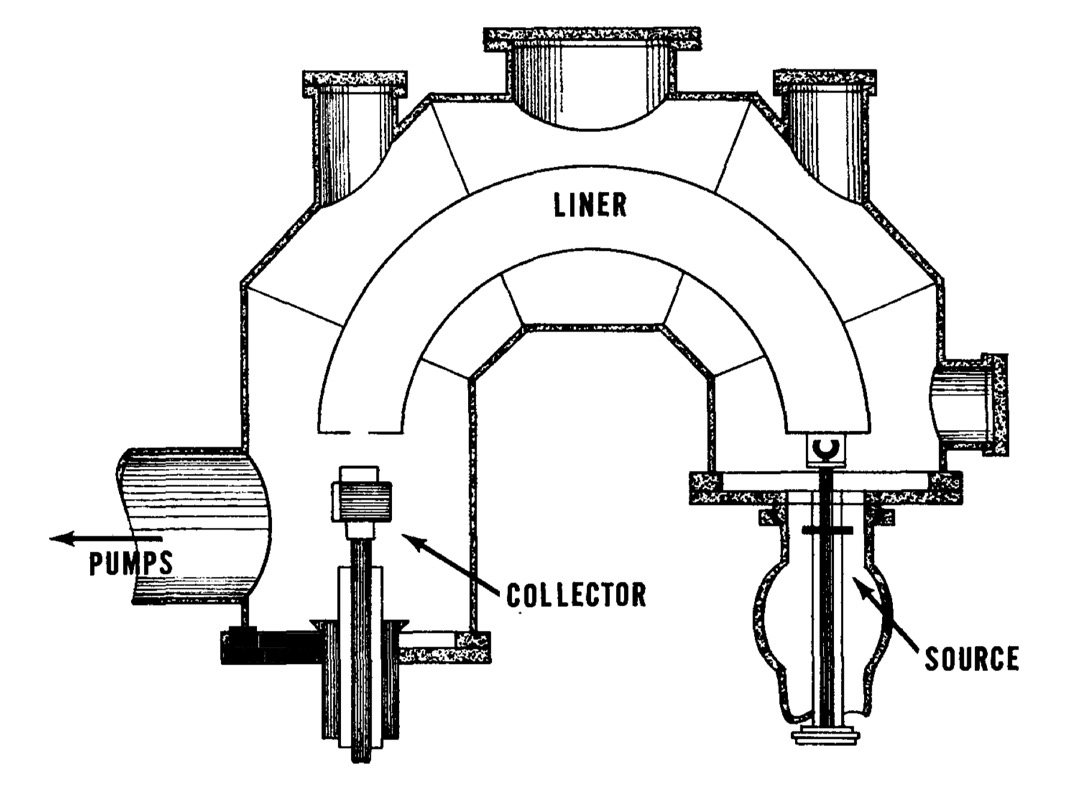
A new 10 milli-ampere source was developed. Two narrow slits focussed the beam, and the 'unwanted' ion's were captured in the box structure. The intensity of the captured beams required a water-cooled collector. A water-cooled copper liner inside the tank between the source and collector was insulated so it could be maintained at the same high negative voltage as the accelerating electrode, thus keeping the ion path free from parasite electric fields that might distort the beam. This new unit was called a 'Calutron'. I've read that the name came from CalU for the University of California, and 'tron' a Greek suffix meaning instrument.
The results of Lawrence and the fact that new estimates of the critical mass (closer to the lower end of the range 2 kg to 100 kg) suggested that they might be able to shave 6 months of the time to complete a bomb. Oppenheimer now estimated a critical mass of 2.5 kg to 5 kg, instead of his original 100 kg. He also estimated a yield of 6% rather than the original 2% (yield in the sense of mass converted into energy).
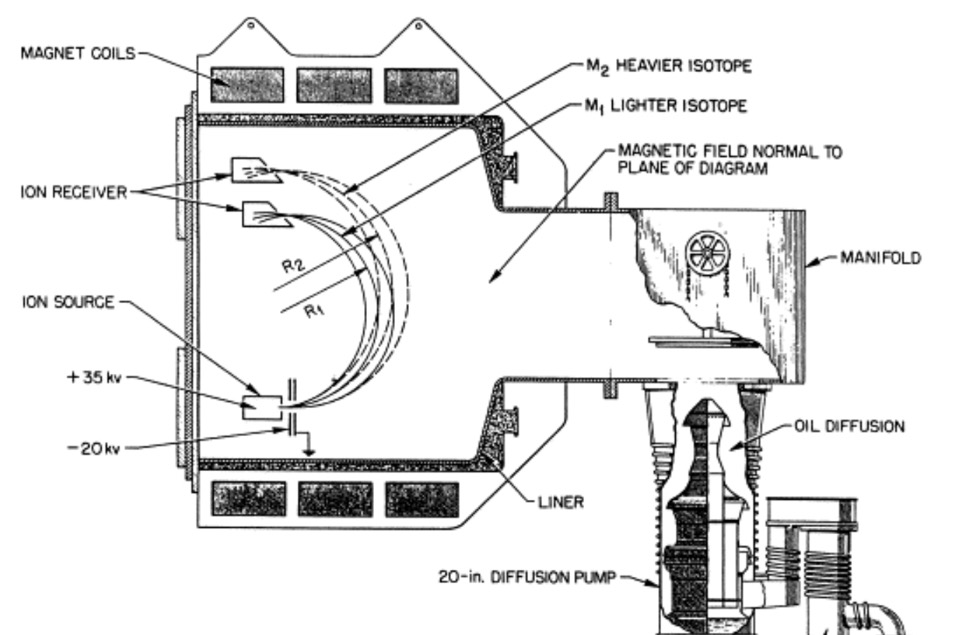
Let's look in a bit more detail how a calutron works (above we had the so-called Beta-calutron but it might help understand better how the machine works). We have ion's passing between the poles of a magnet. A mono-energetic beam of ions of naturally occurring uranium will split into several streams according to their momentum, one per isotope, and each characterised by a particular radius of curvature in the magnetic field. The diameter of an ion's trajectory depends on the strength of the magnetic field, and the mass, electric charge, and speed of the ions. Lighter ions are more easily deflected by a magnetic field, and consequently have a tighter trajectory (smaller diameter). Between uranium-235 and uranium-238 the diameter of the 235U+ ion beam is about 0.6% smaller than that of the 238U+ ion beam. In an electro-magnetic isotope separator with a beam trajectory diameter of 3-4 m, the difference in beam trajectory diameters for the two ions is about 0.7-0.8 cm. To properly focus these beams on their respective collector slits the voltages for ion acceleration (30-35,000 eV) and the current for the magnets must be controlled to within 0.1%.
There were many practical problems. They needed to avoid sparking in the ion source. They needed to figure out how to extract the uranium-235. Some documents mention that they finally decided on using graphite collectors that were disposable and could be burned during the chemical recovery of the uranium. The same reports also mention the need to remove the valuable feed material that had condensed (with the chloride) all over the inside of the tank (over 90% of the feed was deposited on the liners). Some reports mention that the solution was to use disposable stainless steel, water-cooled liners. However there is ample evidence indicating that the source, targets and liners were removed in one piece, and that the liners were hand scraped and brushed over larger sinks. After the cleaning the liners, sources and collectors were leak tested and reused. They also needed to scrape clean the slits to keep the beam intensity constant. We mentioned the issue of space-charge. The technique used was to reduce the vacuum so that the positive ion beams collided with gas molecules in the vacuum chamber, creating electrons that tended to neutralise the repulsion within the beams. This was a delicate adjustment because relaxing the vacuum also reduced the beam intensity. On the 184-inch calutron the magnets were typically nearly 2 metre in diameter and weighted between 10-20 tons, each contained about 400 metres of thick copper wire. These magnets consumed about half of the energy consumption of a calutron, and naturally they also needed to be cooled.
Above we have used one of the calutron production units to describe the basic functioning of the isotope separation process, however the transition from the test unit in the 37-inch cyclotron magnet to the industrial-scale beta-calutron was not an easy one.
The test with the 37-inch magnet was sufficiently interesting for Lawrence to look to test a similar system in the 184-inch magnet. On June 3, 1942 they installed and tested two C-shaped flat vacuum tanks inside the large magnet (see below).
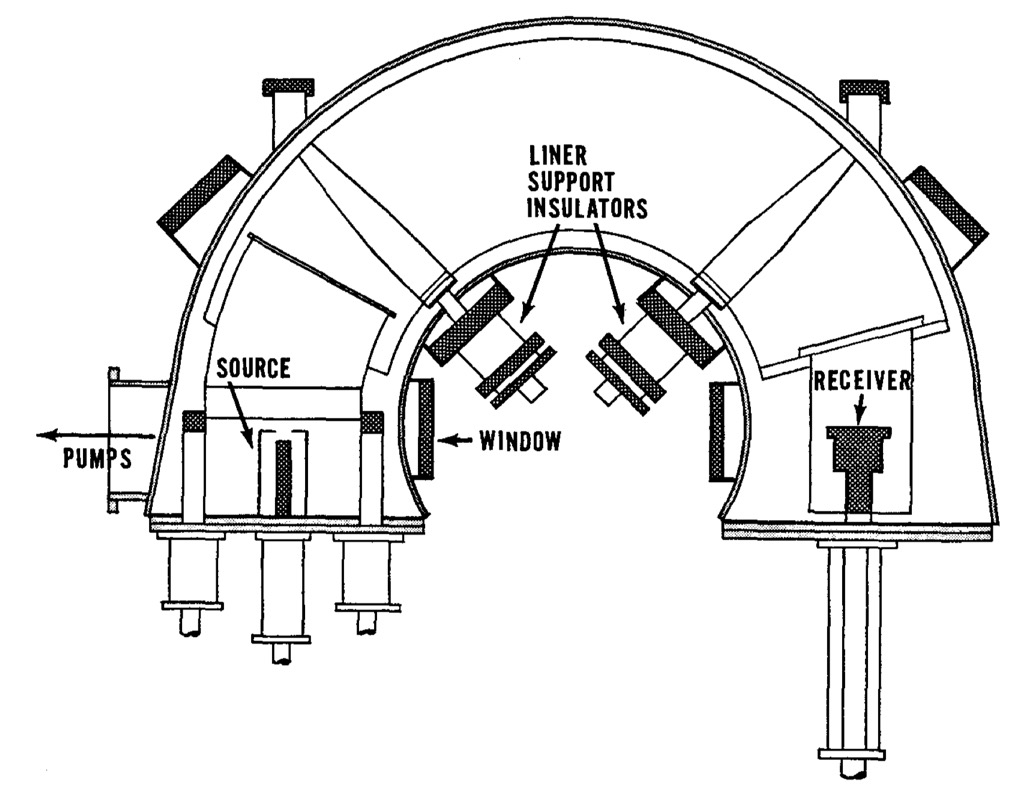
The operator would sit in front of the C-shape, with controls on the left to adjust the ion source. The operator could control the temperature of the heaters which vaporised the uranium chloride, the temperature of the charge chamber, and the power supply to the cathode which ionised the vapour. They could change the position of the cathode or replace it through an air-lock. The operator could also move the arc and the accelerator electrodes in all directions, tilt them, and change the width of the gaps. There was a vacuum-tight window through which the operator could see the adjustments made and the effect on the beam. On the right side were the controls for the collectors. Between the two was the vacuum tank, and an insulated metal liner which could support a very large negative charge.
The above calutron was only tested once for production of uranium-235, the real interest was to understand how to use at the same time several sources in one tank. The trial was with a three-beam unit, each beam about 3 cm apart from the others. It did not produce a definable beam. Nor did trials with just two sources. Single sources worked fine, and two source run at low power could operate together. What was happening was that each source generated a very high frequency oscillation in the other beams. The idea of multiple sources would bring a practical electro-magnetic plant nearer, but understanding the problem did not mean solving the problem. The other tank was used for running focusing experiments and testing different receiver designs.
Building a device that works is one problem, but building an 'industrial' version is another problem. Focus had not been an issue for a test unit, but the reality is that no matter how small the slit was the ions never emerged in exactly parallel paths. So the beam was blurred at the receiver. To solve this, one option is to make slight variations in the magnetic field. This was done by carefully designed contoured metal sheets or 'shims' attached to the top and bottom of the vacuum tanks.
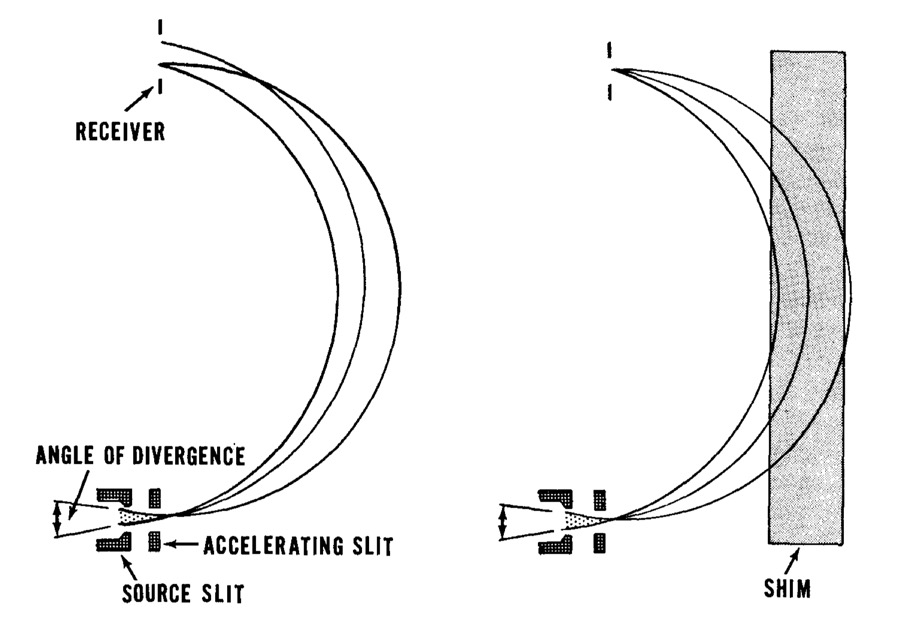
These shims varied very slightly the width of the magnet gap across the pole face. This changed the strength of the magnetic field so 'pulling' the ions in the beam back to focus. It literally took months of trial and error to find the best compromise. The collectors were in fact detectors, and needed to be re-designed so that the uranium-235 could be recovered. The ion beam was intense and could easily burn away a metal or graphite collector. The more intense the beam the more ions would 'bounce out' of the collector. Using multiple sources would need multiple collectors, but there was a problem of beam interaction. The use of shims could be optimised for one ion beam but might adversely affect another ion beam. A new problem emerged and that was the beam of ions no longer struck the face of the collector in a straight line, but as a curve. But this then required slots that were curved. And this was all about trying a design, photographing its effect through a window in the tank, and reshaping the slots for another test. This might have been tiresome but it also revealed a new result. What they found was that the collection was improved when the face of the collector was set at 45° rather than perpendicular to the beam.
The result of all this experimentation was that they knew how to produce a tight beam and they could operate two beams within a few inches of each other. For Lawrence the theory was solid, so the remaining problems were 'just' engineering and the chemical extraction of the uranium-235.
Finally when Lawrence had tested fully the calutron he suggested building 10 of them, to produce 4 grams of uranium-235 daily. The S-1 Committee over-ruled him and recommended spending immediately $12 million to build a plant for 25 times that capacity to be operational before autumn 1943 (a different report mentions a five tank pilot and a full-size plant with 200 tanks). Everyone knew that the alternatives would be better, but in mid-1942 the calutron was the only thing that worked. From end 1943 50 new calutrons were installed monthly, and by the time the war ended Y-12 would house 1,152 calutrons operated by hundreds of women working shifts 24/7. The women operators had to watch the measured beam currents and make minimal adjustments to operating voltages and tank pressures. Women did the job properly, whilst men tended to constantly fiddle with the controls.
Twenty-five years after the end of the war there was an anniversary meeting held at Oak Ridge. General Leslie R. Groves noted that at the time no one thought that electro-magnetic separation would work, and the only reason they continued was because it was backed by Lawrence. And he delivered, even if later gaseous-diffusion delivered larger quantities of uranium-235 and the Y-12 plant was closed down. Groves note that they had in fact kept working two alpha and two beta calutrons for isotope separation and research, and they had delivered over that 25 years more than 200 kg of enriched isotopes for science and medicine.
Isotope Separation - who decided what, and how?
Later on this webpage we will see some of the major decisions made concerning isotopic separation, within the context of the different plants built in Oak Ridge. Below is a kind of experiment, in that I've tried to tie those decisions together in a rough time-line so we can see better the 'play' of one isotope separation technique against another, and how they all came together in the end.
After a meeting on the March 16, 1939 between Pegram of Columbia and the Navy, they contacted Beams about using a centrifuge for isotope separation. It was on May 21, 1940 that Kistiakowsky suggested gaseous-diffusion as a possible means of isotope separation. It was in spring 1941 that Urey took command of research on gaseous-diffusion. It was in 1941 that it was suggested that the electro-magnetic separation method could yield a few grams of uranium-235.
During the spring and summer of 1940 it was gas thermal-diffusion that first attracted the attention of scientists. The idea is simple, molecules in a mixed gas will concentrate in either the hot or cold regions of a container. But the specialists decided that it was impractical for large-scale separation. It was Philip Hauge Abelson who thought to try liquid rather than a gas. He received continued funding by the Naval Research Laboratory.
By April 1941 progress on the centrifuge appeared to be good. Tests in principle were positive, and the first trials with gaseous uranium compounds achieved a degree of concentration of uranium-235. A body of theory was established by Karl Paley Cohen and a 36-inch design was developed. In July 1941 $95,000 was allocated to the centrifuge, $25,000 to gaseous-diffusion, and $167.000 for chain reaction work.
It was in July 1941 that Lawrence decided to convert the 37-inch cyclotron to a giant mass spectrometer, and the calutron and electro-magnetic separation was born (even if Lawrence's initial plans was just for sample preparation).
On November 6, 1941 a report by the National Academy of Science concluded that gaseous-diffusion looked feasible, even if it might be slow to develop. The centrifuge method appeared practical and was further advanced. They also stated that it might take three or four years, and the separation process would be the most time-consuming and expensive part. They estimated that it would cost $50 million to $100 million, plus $30 million to produce the bombs. The report did not mention using element-94.
However in December 1941, the centrifuge had become the second best approach to isotope separation, overtaken by gaseous-diffusion (even if it would be more expensive and may take more time to build a plant). The MAUD report in mid-1941 had clearly sided with gaseous-diffusion for isotope separation. Some reports mention an allocation of $400,000 for Lawrence and electro-magnetic separation, largely based upon the very positive early results with the modified 37-inch cyclotron.
In December 1941 during a re-focusing of the research, Urey took charge of separation by both the gaseous-diffusion and the centrifuge methods, as well as work on heavy-water. In early 1942 experiments with the different separation techniques showed that they worked for lighter elements where the relative difference in weights was large. However no one method seemed to have the capability of the mass spectrometer (electro-magnetic separation) to produce large quantities of uranium-235 in the short time available.
The reality as one author put it was that the "conclusions … were based upon great masses of postulates held together by a thin thread of experimental fact". In fact decisions were not being made on new knowledge, but simply on a greater confidence in the hastily drawn conclusions made in late 1941.
In January 1942 it was decided to build at least one experimental centrifuge and one gaseous-diffusion unit of industrial size. The also decided to design pilot plants using a number of separative units to demonstrate feasibility. Finally they also need to work to secure adequate supplies of uranium oxide, metal and hexafluoride.
Columbia University already had a development contract for centrifuges with Westinghouse, so they were asked to obtain the parts for 24 36-inch units for a pilot plant. The Westinghouse units were scaled up versions of a 7.2-inch laboratory model. Assuming a working centrifuge, a plant producing 1 kg of very pure uranium-235 would need between 40,000 and 50,000 36-inch centrifuges. Standard Oil Development Company was contracted to study the building of a full-scale plant. The Columbia group were also looking to a 132-inch centrifuge and an initial plant of 624 123-inch centrifuges in a 15-stage cascade. But for a production of 1 kg/day they would still need about 10,000 of these large centrifuges. An alternative was to focus on 100 gm/day which would 'only' require about 6,000 of the 36-inch centrifuges. Westinghouse said that once they had the order, they could make 1,000 centrifuges a month. In late February 1942 Westinghouse was asked to do the engineering study for a 100 gm/day plant, a decision that had little significance give the problem encountered during the winter of 1942.
The reality was that in early 1942 everyone was beginning to understand that the engineering development of both gaseous-diffusion and the centrifuge would take time.
In early 1942 Beams had completed two runs with uranium hexafluoride through the 36-inch simple flow-through centrifuge. In theory it should have managed an enrichment from 0.72% to 0.73%, but the different was too small to measure. Westinghouse was having to focus on just designing a single industrial scale centrifuge, never mind a pilot plant.
In early 1942 the key problem was developing a metal barrier for gaseous-diffusion. This was such a complex issue that they could not even decide on how to build a large-scale barrier production plant.
Aside from the specifics of each separation process, all of them required large quantities of uranium hexafluoride. Until 1940 only gram quantities were produced, but in 1940 Philip Abelson working at the Naval Research Laboratory found that he could easily and safely produce uranium hexafluoride by fluorinating uranium tetrafluoride rather the metallic uranium. By the spring 1942 a pilot plant was operating, and a rapidly expanding production was predicted.
By May 23, 1942 neither team had produced a single operating centrifuge or gaseous-diffusion unit of any practice size. No 'short-cuts' had been found, and there was no way to 'pick-a-winner'. On the other hand Lawrence was expecting to have firm evidence on electro-magnetic separation (calutron), enough to justify a full-scale plant. No one was able to draw any conclusions about the relative merits of gaseous-diffusion and the centrifuge. Maybe this would change once pilot plants were built and tested, but that would take another 6 months. And no one needed a plant making 100 gm/day. They needed to design and build a 1 kg/day plant. They finally decided to build a 100 gm/day centrifuge plant for January 1944, at a cost of $38 million (it was never built). They decided to build a pilot plant for gaseous-diffusion and look at the engineering for a production plant. And they decided to build a 100 gm/day electro-magnetic plant to be completed by September 1943 at a cost of $12 million. Added to $25 million for the piles for the production of element-94, the total budget was $80 million (plus $34 million annual operating costs). The target was a 'few atomic bombs' by July 1944, and about twice as many each year thereafter. Deciding all this was one thing, convincing the Army was another. They agreed to the work on the piles and electro-magnetic separation, but concerning gaseous-diffusion and the centrifuges they decided not to decide.
In July 1942 people were increasingly interested in the progress made on electro-magnetic separation, and the question if that progress justified a full-scale electro-magnetic plant. Another question was the possible feasibility of producing element-94.
During the period May-October 1942 Beam had succeeded in running a sample of uranium hexafluoride three times through his experimental centrifuge, proving that enrichment was feasible. However when the samples were analysed they were only about 60% of the predicted enrichment. Looking back this alone should have been enough to kill this option. For the 100 gm/day plant the first estimate was for 8,800 machines, but based upon the laboratory trial the number of machines needed rose to 25,000. Also the hold-up of uranium in the plant rose from 4 months to 1 year. There were two remaining options. The first was to improve the build-quality of the machines. The second was to drop the flow-through principle (used in the laboratory) in favour of a theoretically more efficient but more complex counter-current design. This technique would reduce the number of machines by 20% and the machines themselves would be smaller. Trials were started, but at the same time Westinghouse was having problems testing the 36-inch flow-though model. There were severe instabilities at critical vibration frequencies. On top of that the gas-tight, corrosion-proof seals were not working properly. There were frequent failures of the motors, shafts, and bearings at high speeds. The way Beams had designed the centrifuge it consisted of a coaxially mounting between two drive shafts. The tube was then surrounded by the critical vacuum casing, and the drive shafts protruded through openings in the casing. The protrusions were made vacuum-tight with heavy-grease seals. The ends fo the drive shafts were then supported outside the case with conventional rigid bearings. The seals and bearings resulted in a tremendous loss of energy in the form of heat. They were losing about 1 kW as compared to a 1 W for a modern centrifuge. Friction meant that these components simply wore out quickly. This type of equipment was fine for short laboratory trials, but was totally inappropriate for industry-scale operation. The 36-inch machines were delivered on May 29, 1943 and started operation in August 1943, but by September 10, 1943 there were problems. Westinghouse still continued with a pilot plant in Bayonne, New Jersey, but they needed to solve all the problems for the 24 centrifuge pilot plant. By October 1943 the experimental counter-current centrifuge built in Virginia had worked for only short periods, and it was difficult to see how a reliable unit would be available in the near future. Many of the engineers in the different teams were not sure that the problems could be solved and a full-scale plant built.
During a meeting on October 26, 1942 it was evident that none of the separation techniques came out on top, but the centrifuge was definitely the weakest. Gaseous-diffusion looked feasible, but would probably not be completed in time (i.e. for July 1944). Neither technique was likely to deliver before early 1945. The need for a gaseous-diffusion pilot plant that would take a year to build and test was questioned. The pilot would not have produced useful quantities of uranium-235. A full-scale plant could be completed almost as quickly as the pilot. Progress in Chicago with the pile was good, and full-scale production was planned for spring 1944. So Lawrence's electro-magnetic technique was the best bet. It probably could deliver a kilogram by January 1944, and they could see that it could produce 100 gm/day of uranium-235 through 1944. But the requirements were just staggering to everyone, including Lawrence. Even so, Lawrence promised that there were no fundamental difficulties to build a large-scale electro-magnetic plant.
During the period September 28, 1942 to November 10, 1942 things moved quickly. It was agreed that the electro-magnetic technique would go directly to a full-scale plant, bypassing the pilot plant. They had already decided to do the same for gaseous-diffusion, so it was time to eliminate the centrifuge development. And Stone & Webster and Du Pont would develop the full-scale pile and electro-magnetic plants. Kellogg would continue on the gaseous-diffusion, with a view to a 600-stage plant.
So the priority was on the electro-magnetic technique, then gaseous-diffusion, and finally the pile (and plutonium separation). Laboratory work would continue on the centrifuge and 'isotron' (a device that, unlike the calutron, used an electric field rather than a magnetic field to separate uranium isotopes).
On December 2, 1942, the Lewis Committee reported that they were impressed by the possibilities of gaseous-diffusion, but there were less impressed by the partnership Kellogg-Columbia. At the same time Compton was promising a bomb with element-94 (plutonium) in 1944 (even if at this moment Fermi had not yet demonstrated a chain reaction). The Lewis committee actually concluded that gaseous-diffusion had the best chance of success, and they recommended the design and construction of a full-scale gaseous-diffusion plant. The committee also recommended to continue work on the calutron, but with the equipment available a full-scale plant would require 22,000 calutrons, which was considered impractical. But they did recommend building a small plant with 110 calutrons in order to have uranium-235 samples for testing.
Bush sent a report to Roosevelt on December 16, 1942 concerning the bomb. The centrifuge had been eliminated, and the recommendation was to build a full-scale gaseous-diffusion plant for $150 million, plutonium plants for $100 million, and a small electro-magnetic plant for $10 million.
The key decision after December 16, 1942 was to try to transfer design responsibilities from the universities to experienced engineering companies, for example the electro-magnetic plant had been reduced to 500 tanks so that it could be built as fast as possible, but Stone & Webster could order all the material they needed for the full-sized plant (responsibility had moved from Lawrence to the Army and then to Stone & Webster as prime contractor). Groves also contracted Union Carbide to build the gaseous-diffusion plant. After this decision things started to move faster on the electro-magnetic plant. Westinghouse had to make the tanks, liners, sources and collectors, General Electric the high-voltage equipment for the magnets and tanks, and Allis-Chalmers for magnet coils. The planning was to deliver 50 tanks a month, and to have all 500 working by the end of 1943.
Oak Ridge would be home to Y-12 (electro-magnetic) plant, X-10 (plutonium-producing pile) and K-25 for gaseous-diffusion. By early 1943 the cost of this site had risen to $492 million.
In January 1943 a different approach was re-discussed. The idea was to eliminate the upper stages of the gaseous-diffusion plant (a so-called 'squared-off' cascade) and substitute those upper stages with either centrifuges or calutrons. This decision was finally made in August 13, 1943, and the gaseous-diffusion plant would be limited to 50% enrichment, and would feed electro-magnetic enrichment in Y-12. Given that the ground had not yet been broken on the building of K-25, it was also decided to double electro-magnetic enrichment in Y-12.
In February 1943 having seen the centrifuge option abandoned, work continued on improving the reliability of the machines. The option that the centrifuge might be used to 'top-off' enrichment from a gaseous-diffusion plant was kept open until September, when it was decided in favour of a 'top-off' with electro-magnetic machines (e.g. K-25 feeding Y-12).
During 1943 Urey had become increasingly pessimistic about gaseous-diffusion. Why had they continued to work on the Norris-Adler barrier when it had proven to be unsatisfactory? Urey was of the opinion that war projects should not rely on a major research effort, and by this time there were 700 people working on gaseous-diffusion in Columbia (and several hundred people elsewhere). Urey thought that they should not spend more money on this technique, and not build K-25. On the positive side there was some progress in make a nickel powder barrier, and the cascade design proposed by the British looked impressive. Groves had in any case decided to complete K-25. The investment already committed was just too big and Kellex (the K-25 contractor) already had 900 people working on the plant design. They had invested $5 million is a plant for the production of the barrier, and $4 million in a plant for the pumps.
Groves replaced Urey with Lauchlin M. Currie from the National Carbon Company, and Hugh Stott Taylor continued the work on the new barrier. Thus Urey was removed from the gaseous-diffusion project, one that he believed would surely fail.
If gaseous-diffusion (K-25) was to deliver in 1945, then the barrier would need to be produced by summer 1944. A meeting on November 5, 1943 offered some hope that the new nickel-powder barrier would deliver, despite the Army still planning to produce the older option. And by January 4, 1944, Union Carbide had agreed to take on the new barrier. This decision meant setting aside 2 years work on the old Norris-Adler barrier, and committing K-25 to the new barrier. One of the advantages of the new barrier was that it was simple to produce by hand, but it would need thousands of people doing simple batch work. Although it did imply ripping out the machines for producing the old barrier, and installing a new process. Summarising - they would build a fabrication facility for an untested barrier in four months, and produce all they needed in the following four months, and have K-25 running within a year.
Changing to the new barrier did not mean abandoning the older Norris-Adler barrier, it just meant that it stayed in the laboratory. In fact, with a new team, results with the older barrier started to look more promising, even if it required a much more complicated manufacturing process than the new barrier. By April 1944 it looked as if they had two different barriers made in two different laboratories. In early 1944 only about 5% of the new barriers were within the specification, but by April 1944 it was up to 45%. By June 1944 they were producing pumps, coolers, compressors and converters by the thousands. And they had about a half million valves and about 1 million metres of piping ready. Already in April 1944 six stages had been installed in K-25, all without the barriers. At that time nearly 20,000 people were working on K-25, and the cost had climbed to $281 million.
As the contractors for the electro-magnetic plant started design work they were able to introduce modification that would double the capacity of the 500 tanks. But going from the laboratory to a full-size plant also meant that changes affected other decisions that everyone thought were final. The tanks grew from 3 meters to over 4 meters in height. When the use of two sources had been accepted, Lawrence started to look at four, eight or even sixteen sources. Some of Lawrences team supported the idea of two types of calutrons, one using the enriched material from the first five 'racetracks'. On March 17, 1943 they decided to stick with the basic calutron design, but the idea of two different types of calutron won through. The calutrons under construction were re-named 'Alpha' and the second stage calutron was called 'Beta'. The five 'Alpha' racetracks would feed only two smaller 'Beta' racetracks. The two magnets would contain 36 tanks, and 72 sources. There were a lot of positives. The 'Beta' racetracks were smaller, could be pumped down faster, and would have shorter run times. The down side was that a single loss in a 'Beta' would mean losing several 'Alpha' runs.

Later we will again come across 'racetracks' in Y-12. Above we can see an Alpha 'racetrack' with some calutron tanks sitting in the bottom left corner. And below we can see the general layout of a 'racetrack' taken from patent. We see that it is a closed series of alternated tanks (no.11) and electro-magnets (no.12). The electro-magnets consist of a winding enclosed in a case (no.14) and surrounding a core which is made up of honeycombs or grids. The electro-magnets in the curved region comprise a pair of windings each is a separate winding case (no.17) with a portion of the wedge-shaped core exposed (no.18). The mid-yoke (no.20) is a beam of iron, and in order to prevent distortion of the field the end portions (no.21) are specially shaped.
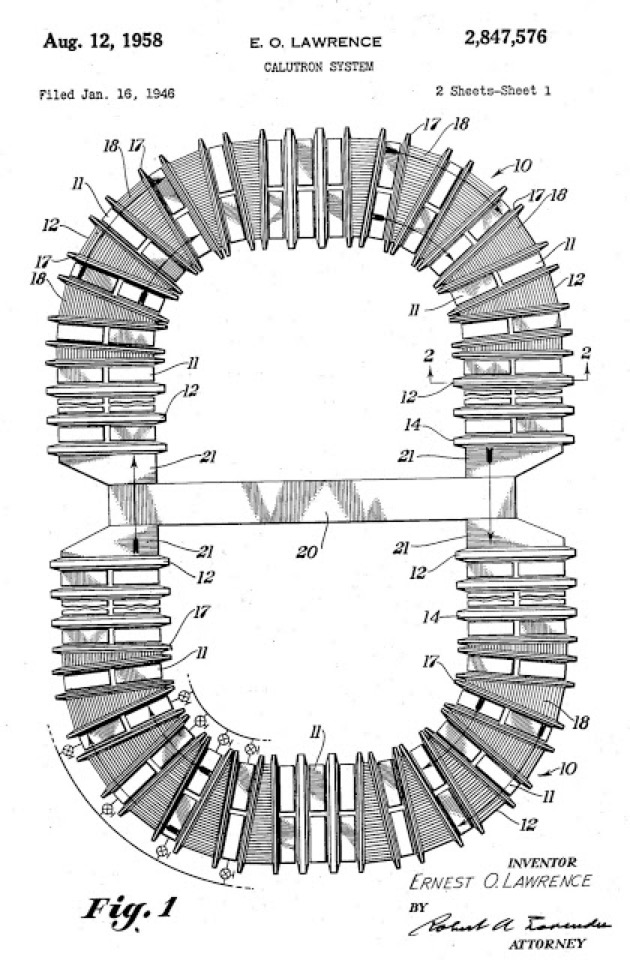
One 'Alpha' racetrack was tested in August 1943, but it was not sure that all five racetracks would be working by the end of 1943. However, Oppenheimer emerged from Los Alamos with a three-fold increase in the amount of uranium-235 that would be need for a bomb. Would Y-12 ever produce enough material for a weapon? On the other hand the problems with the barrier for the gaseous-diffusion process meant that the electro-magnetic process was the only game in town. Maybe it could deliver enough uranium-235 for a bomb before the end of 1944. It was at this moment that the idea to eliminate the top cascades of K-25 and feed Y-12 was introduced. Naturally Lawrence used this option to argue to increase the electro-magnetic plant.
In December 1943 it was decided to let the centrifuge project run to its conclusion, but not to renew the contract. The best they had managed was to run one centrifuge for 99 days before it broke down.
Through 1944 Groves followed the idea that K-25 would feed the electro-magnetic separators in Y-12. If K-25 only provided a low enrichment, then this would feed into the 'Alpha' racetracks. But to do this the 'Alpha' machines would need different receivers. More importantly a lot more care would be needed in cleaning the 'Alpha' liners and recovering the now valuable charge in uranium-235 obtained from K-25. More importantly the latest design of the 'Alpha' racetracks had 'simplified' things, i.e. expecting to only work with natural uranium the liners had been removed, and only the source and receivers were to be washed. Now they would have to be completely redesigned, and the washing area doubled in size. The recovery process would need to be optimised and the risk to accumulate a critical mass in the process would need to be eliminated. Lawrence had already argued to expand the 'Alpha' to nine racetracks, and now he argued to add another four racetracks.
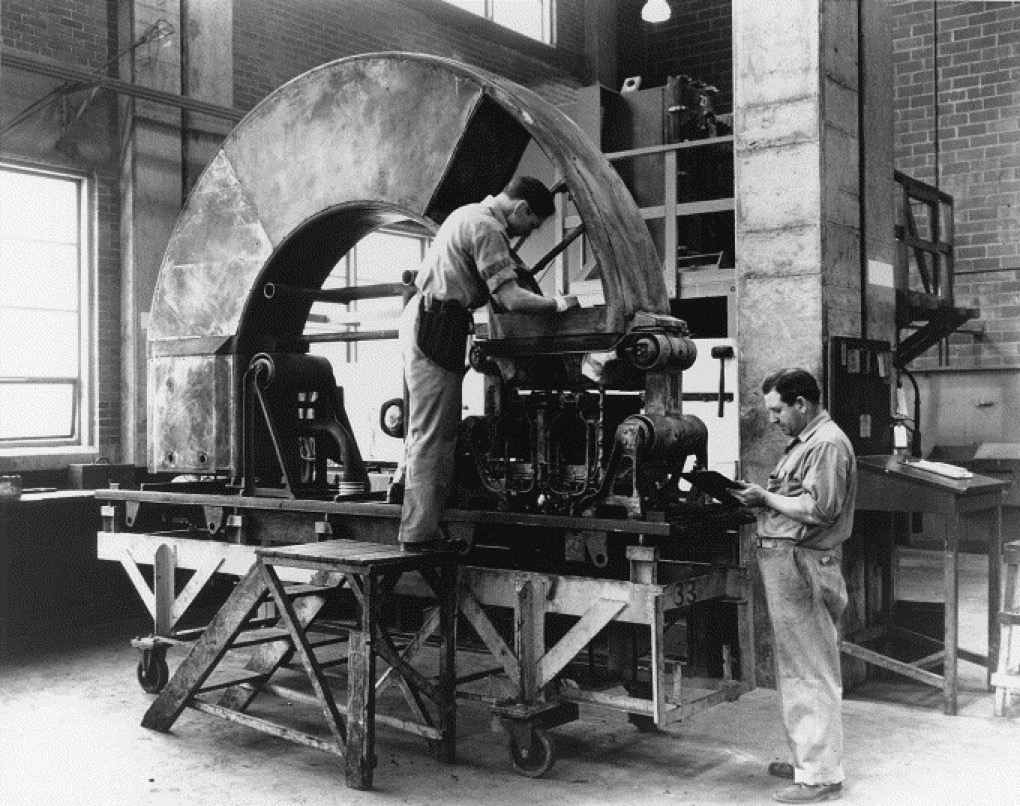
Above we can see the liners being washed, and below the collection slits from the Beta calutron for one isotope pair being cleaned and replaced.

On March 30, 1944 there was a meeting with all the major actors, and as can happen, each went away with a different opinion on what was decided. To some it looked as if gaseous-diffusion would feed Y-12 and no more electro-magnetic racetracks were needed, to others it looked as if uranium-235 had almost been dropped in favour of plutonium. Lawrence thought that K-25 might never work, so to him Y-12 was the only answer. His idea was to upgrade the existing 'Alpha' model to four beams and the new model to eight beams, and build 2 more 'Alpha' racetracks. On June 30, 1944 it was decided not to modify the existing 'Alpha' racetracks, but the new 'Alpha' racetracks would be completely redesigned with a cold source and 30 beams. Many people thought that even a combination of K-25 and Y-12 would not produce enough material for a bomb in 1944, or even early 1945.
At this point we will need to 'back-track' a little in time and again look at the work of Philip Abelson at the Naval Research Laboratory on liquid thermal diffusion. Firstly this is an example of what is called thermophoresis, a phenomenon where in a mixture of different particles types they will respond different to a temperature gradient. The effect was first reported on in 1856 and Charles Soret gave his name to the effect in 1879. The Chapman-Enskog theory explains that when a mix of two gases passes through a temperature gradient the heavier gas tends to concentrate at the cold end and the lighter gas at the warm end. The was used by Klaus Clusius in 1938 to separate the isotopes of neon. Abelson became interested in the use of liquid thermal diffusion to separate isotopes, and he was able to achieve a separation factor of 20% between potassium-39 and potassium-41.
Moving to uranium Abelson found that hydrolysis affected uranium salt solutions (spontaneous formation of ions), so his only option was to work with uranium hexafluoride. He received funding to continue his work, but at the time it was felt that the process would not scale to an industrial plant. Because uranium hexafluoride was not readily available Abelson devised a method to produce it in quantity. His small plant provided the samples for the work in both Columbia and Virginia, and his design was used to produce the uranium hexafluoride for the weapon program.
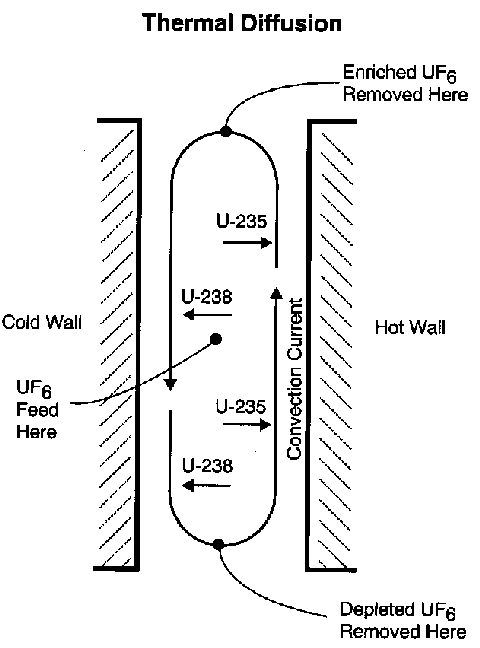
Liquid thermal diffusion is simple, uranium hexafluoride is introduced under pressure into the annular spacing between two concentric vertical pipes. The outer wall is cooled and the inner heated by high-pressure steam. The lighter isotope will concentrate near the hot wall and the heavier near the cold wall. Convection will carry the lighter isotope to the top of the column. The taller the column the greater the separation effect.
Abelson continued his work at the Naval Research Laboratory and he successfully tested the process on June 22, 1942 (a pilot plant was authorised in July 1942). Oppenheimer read the reports of Abelson, but he was convinced that it would be impossible to produce fully enriched uranium-235 by this method. By July 1944 Abelson was able to produce 5 gm/day of 5% enriched uranium. Oppenheimer now saw that the plant could actually be scaled to 100 columns operating in parallel and produce about 12 kg/day at 1% enrichment.
Early on Roosevelt had decided to exclude the Navy from the weapons development, so Abelson's work on liquid thermal diffusion was more or less ignored. There had been some contact but the work of Abelson looked less promising (at the time) than the electro-magnetic separation and the pile for element-94. And the results obtained in Columbia and Chicago had not been given to the Navy. It was in December 1942 that they learned that Abelson had two 16 metre tall columns processing 2 kg/day of uranium hexafluoride with a significant separation. Abelson had improved the performance of his system by simply increasing the temperature difference between the two walls. The principle was very simple, had no moving parts and no valves, and was relatively inexpensive to build, but on the negative side the process took a very long time to come to equilibrium and produce enriched uranium (a large plant might take up to 600 days to reach equilibrium for fully enriched uranium). As has been noted this performance would have put it 'on par' with the gaseous-diffusion and centrifuge projects in 1942, but in 1943 it looked less positive.
But if liquid thermal diffusion were to be limited to 10% enrichment, then it could be considered as good as the gaseous-diffusion process planned for K-25. So why not replace gaseous-diffusion with liquid thermal diffusion? On February 10, 1943 it was decided to go ahead with preliminary engineering studies on liquid thermal diffusion, but the relationship between the Manhattan Project and the Navy would breakdown in the summer of 1943.
However Abelson continued his work, and it was in April 1944 that Oppenheimer again suggested that liquid thermal diffusion might be of value. The key to the process of Abelson was to increase the temperature difference between the hot and cold walls. In November 1943 he obtained the agreement of the Navy to build a 300 column plant in the Naval Boiler and Turbine Laboratory in Philadelphia, and by June 1944 he had build a 100 column test plant. In early 1944 it was agreed that the Army and Navy could (again) cooperate. More or less immediately Groves signed an agreement to build 21 exact copies of the Abelson 100 column test plant. Despite the fact that there were still problems with the barrier for the K-25 gaseous-diffusion plant, they had built in Oak Ridge a power plant that was ready to produce the necessary steam for the liquid thermal diffusion columns. The entire plant could be up and running within 90 days (starting June 27, 1944). This new plant was called S-50.
Making 100 16-metre tall columns was one thing, making 2,000 of them was another. The inner pipe used for the liquid thermal diffusion column was in nickel and the outer in copper, and the gap was only 0.005 cm. Within 69 day they had 320 columns working, whereas the K-25 gaseous-diffusion plant was not yet finished, and they had received just two complete converters of the 1,000's expected, and tests were still being performed on dummy barriers.
By March 1945 S-50 and Y-12 were working. All 21 racks in liquid thermal diffusion plant (S-50) were working and feeding the electro-magnetic plant (Y-12). The precise operating characteristics of the gaseous-diffusion plant (K-25) were not yet known. S-50 was enriching from 0.71% to 0.89%. This was fed to the Alpha racetracks in Y-12, then to the Beta racetracks. When K-25 came on line the feed from S-50 would go to K-25 to be enriched to 1.1% before being sent to Y-12. Over time K-25 would be brought up to 20%, and the Alpha I calutrons in Y-12 would be phased out.
In April 1945 the Alpha II racetracks were working on 1.1% material obtained from S-50 and K-25. It was finally decided to stop shipping from S-50 to Y-12, and ship from S-50 to K-25. Finally the gaseous-diffusion plant was working. By June 1945 K-25 was supplying 7% enriched material directly to the Beta racetracks in Y-12.
Not strictly in chronological order, but throughout 1944 Groves was under pressure to commit to a long-range construction plan. He finally decided to build a second gaseous-diffusion plant (K-27) and to add a fourth Beta electro-magnetic racetrack to Y-12. Both were planned for operation in 1946. At that time Groves assumed was that the war with Japan would not be over before mid-1946.
By August 1945 K-25 was fully operational, and by January 1946 K-27 was operational. What became evident was that K-25 and K-27 were now able to obtain the same enrichments as the Beta racetracks in Y-12. The problem of a potential holdup of a critical mass in K-25 was now a problem, and another problem was the speed with which corrosion was spreading in the plant was an unknown.
The above section has tried to tie together the different developments concerning isotope enrichment and separation, but now we must return to our chronological view of events, and we do so on the day the U.S. entered WW II.
The U.S. Enters the War
Following the Pearl Harbor attack on December 7, 1941, the United States entered WW II (Germany and Italy declared war on the U.S. three days later).
In January 1942, President Franklin D. Roosevelt gave secret, tentative approval for the development of an atomic bomb. The Army Corps of Engineers was assigned the task and set up the Manhattan Engineer District to manage the project. A bomb research and design laboratory was to be built at Los Alamos, New Mexico. Due to uncertainties regarding production effectiveness, two possible fuels for the reactors were to be produced with uranium enrichment facilities at Oak Ridge, Tennessee, and plutonium production facilities at Hanford, Washington.
Just before the attack on Pearl Harbor Compton’s committee had concluded that a critical mass of between 2 and 100 kilograms of uranium-235 would produce a powerful fission bomb and that for $50-$100 million isotope separation in sufficient quantities could be accomplished. Bush forwarded the findings to Roosevelt under a cover letter on November 27, 1941. Roosevelt did not respond until January 19, 1942 when he did, it was as commander in chief of a nation at war. The President’s hand-written note read,
“V.B. OK returned
I think you had best keep this in your own safe
FDR”.
In 1942 it had been estimated that military and industrial targets in Germany could be devastated with 500,000 tons of TNT bombs, which would be the equivalent to 1 to 10 tons of uranium-235. It was said that Bush had instructed everyone not to discuss the expense of producing uranium-235 to avoid arousing government fears of excessive costs.
As already mentioned, with the written agreement of Roosevelt Bush immediately put Eger Vaughan Murphree, a chemical engineer with the Standard Oil Company, in charge of engineering studies and supervising pilot plant construction and any laboratory-scale investigations. He confirmed Urey, Lawrence, and Compton as program chiefs. Urey headed up work including diffusion and centrifuge methods and heavy-water studies. Lawrence took electro-magnetic and plutonium responsibilities, and Compton ran chain reaction and weapon theory programs.
Following this Compton decided to concentrate the work for which he was responsible at the University of Chicago. The Columbia group under Fermi, and its accumulated material and equipment, and the Princeton group, which had been studying resonance absorption, were moved to Chicago in the course of spring 1942.
From the meeting of December 18, 1941 Compton decided that they needed to demonstrate a chain reaction within 6 months. For the exponential piles and measurements of physical constants he wanted 80 men and $340,000 for Columbia and Princeton, and for Chicago he wanted 58 men and $278,000. He also wanted $500,000 for pile materials. Before Pearl Harbor these figures would have been fantastic, after, they became reasonable. In a meeting of his new group on January 3, 1942 he found that his three teams could not agree on anything. Finally they did agree on the experimental data for the 'pile' but not on specifications. Oppenheimer's name again appeared as the one to coordinate paper studies on fast-neutrons. Another meeting on the January 18, 1942 made everyone realise that each had made some mistakes (including Fermi underestimating the poisoning effect of boron). A plan was outlined in which a bomb would be ready in early 1945. They also decided on the purchase of 30 tons of uranium metal. All the teams had argued for research to be concentrated on one site. Compton finally decided to bring Columbia and Princeton to Chicago. For space he appropriated part of the cyclotron room (in the Service Building), most of Eckhart Hall, and the area under the north and west stands of Stagg Field (American football fields).
Under Lawrence the investigation of large-scale electro-magnetic separation was accelerated at the University of California at Berkeley and a related separation project was started at Princeton.
Before the meeting of December 18, 1941 Lawrence had already started to convert the 37-inch cyclotron to a mass spectrometer.
Research and development on the diffusion process and on the production of heavy-water continued at Columbia under Urey, under the general supervision of Murphree. The centrifuge work continued at the University of Virginia under Beams while the Columbia centrifuge work was transferred to the laboratories of the Standard Oil Development Co. at Bayway, New Jersey.
With the entry of the U.S. into war the situation changed from having too little money and no deadlines, to a situation with a clear goal, plenty of money, and too little time.
On March 9, 1942, Bush sent a report to the President reflecting general optimism but placing proper emphasis on the tentative nature of conclusions. Compton had promised a multiplication factor of 1.14, but they had overestimated the expected results with uranium metal and the projected first pile experiment suddenly grew by 15%. Fortunately this discouraging information came too late to influence the decision of Roosevelt. The report of Bush contemplated completion of the project in 1944. In addition, the report contained the suggestion that the Army be brought in during the summer of 1942 for construction of full-scale plants.
The entire heavy-water program was under review in March and April 1942. The reviews followed a visit to the United States in February and March 1942 by Francis Simon, Hans Heinrich von Halban, and Wallace Alan Akers from England. In a memorandum of April 1, 1942 addressed to Bush, Conant reviewed the situation and reported on conferences with Compton and Briggs. His report pointed out that extremely large quantities of heavy-water would be required for a plutonium production plant employing heavy-water instead of graphite as a moderator. For this reason, he reported adversely on the suggestion that Halban be invited to bring to this country the 165 litres of heavy-water which he then had in England.
In a memorandum written to Bush on May 14, 1942, Conant estimated that there were five separation or production methods which were about equally likely to succeed: the centrifuge, diffusion, and electro-magnetic methods of separating uranium-235, and the uranium-graphite pile and the uranium-heavy-water pile methods for producing plutonium. All the production methods were considered about ready for pilot plant construction and perhaps even for preliminary design of production plants. If the methods were to be pushed to the production stage, a commitment of $500 million would be entailed. Although it was too early to estimate the relative merits of the different methods accurately, it was presumed that some methods would prove to be more rapid and efficient than others. It was feared, however, that elimination of any one method might result in a serious delay.
It was also thought that the Germans might be some distance ahead of the United States in a similar program.
Research on uranium required uranium ore, and obtaining sufficient supplies was the responsibility of Murphree and his group. Fortunately, enough ore was on hand to meet the projected need of 150 tons through mid-1944. Twelve hundred tons of high-grade ore were stored on Staten Island, and Murphree made arrangements to obtain additional supplies from Canada and the Colorado Plateau, the only American source. Uranium in the form of hexafluoride was also needed as feed material for the centrifuge and the gaseous and thermal diffusion processes. Philip Abelson, who had moved from the Carnegie Institution to the U.S. Naval Research Laboratory, was producing small quantities, and Murphree made arrangements with E. I. du Pont de Nemours and Company (their TNX Division for the atomic energy program) and the Harshaw Chemical Company of Cleveland to produce hexafluoride on a scale sufficient to keep the vital isotope separation research going.
In fact in addition to the 1,200 tons on Staten Island the Belgian owners also had 100 tons of oxide at a refinery on the shores of Lake Ontario. Also the Eldorado Mine near the Artic Circle could produce 300 tons per year. And there was at least 500 tons of oxide in waste sludge from vanadium refineries on the Colorado Plateau.
Obtaining uranium metal was problematic. As a powder it is highly pyrophoric and very difficult to melt and cast. In addition there was a supplementary requirement of less than 2 parts per million boron. During 1942 only a few kilograms could be produced at any time.
Finally the centrifuges, gaseous-diffusion and thermal-diffusion processes needed uranium hexafluoride, which until 1940 could only be produced in laboratory conditions. In 1942 the Planning Board contracted for a pilot plant able to produce 3-5 kg/day uranium hexafluoride.
There was also a request for one to two tons of heavy-water, and finally in 1942 a technique was found allowing them to build a pilot plant. Whilst heavy-water would have been the better moderator for a 'pile' they simply did not have enough. With 5 tons heavy-water they could have built a small pile and produce 100's of grams of element-94 (I'm giving up using '….' around pile, and pile, reactor and core all mean the same thing). With 30 tons they could have imagined producing a kilogram of element-94 per week. But with the best will in the world they might have had 5 tons in mid-1943, and 30 tons only in mid-1945. And it must be admitted that at the time the results with graphite were encouraging, the first experiments in Chicago gave a ƙ of 0.94 +/- 0.02. With a similar configuration Fermi also obtained a ƙ of 0.9 in Columbia. The feeling was that by refining the experiment and better quality materials they could obtain a ƙ of more than 1.0. In fact by July 1942 Fermi had obtained a ƙ of 0.995 just by improving the experiment and refining the calculation.
Uranium metal was also required. Uranium ore is usually refined as uranium oxide, often called black oxide, or uranium salt. In early 1942 only small quantities of uranium metal were available. In fact uranium ore had been stockpiled from a mine in northwest Canada, but the mine had to be re-opened when a large new order for uranium ore was made in 1941. The National Bureau of Standards developed a new process for removing all impurities by a single extraction method, and the Mallinckrodt Chemical Works developed a large-scale production unit for uranium oxide. This was then converted into uranium tetrafluoride, or green salt, which is the feed material for most uranium metal-making processes.
Graphite was available in the U.S., but what was needed was a very pure graphite, in particular with no boron. The National Bureau of Standards traced the boron in commercial graphite to the coke used for its production. Coke was substituted with petroleum, which solved the problem.
Heavy-water was another problem. Arrangements had been made post June 1943, but there was an immediate need, and the world’s entire stock of heavy-water (aprox. 400 pounds) was in the hands of the British. In fact Joliot-Curie had sent his collaborators, Hans von Halban and Lew Kowarski, with 165 litres of heavy-water, first to Britain, then to Canada.
There was a major requirement for several thousand tons of copper for the magnet coils used by Lawrence. But copper was a critical war material, so silver was used. Initially when they asked for 15,000 tons of silver from the Under Secretary of the U.S. Treasury, the answer was “we usually talk of silver in ounces”. The first transfer was for 175 million fine troy ounces, i.e. 6,000 tons, but the final figure turned out to 14,700 tons.

This photograph appears in numerous publications, but only a few mention that what we are looking at are the silver coils waiting to be picked up after Y-12 had been closed down.
Conant emphasised a question that has been crucial throughout the development of the uranium project. The question was whether atomic bombs would be decisive weapons or merely supplementary weapons? If they were decisive, there was virtually no limit to the amount of effort and money that should be put into the work. But no one knew how effective the atomic bombs would be.
Lawrence proved to be very successful in producing enriched samples of uranium-235 electro-magnetically with his converted cyclotron. Bush told the President that Lawrence’s work might lead to a short cut to the bomb, especially in light of new calculations indicating that the critical mass required might well be smaller than previously predicted. Bush also emphasised that the efficiency of the weapon would probably be greater than earlier estimated and expressed more confidence that it could be detonated successfully.
The President responded: “I think the whole thing should be pushed not only in regard to development, but also with due regard to time. This is very much of the essence”.
In May 1942, Conant suggested to Bush that instead of encouraging members of the section individually to discuss their own phases of the work with Conant and Briggs, the OSRD S-l Section should meet for general discussions of the entire program. Bush responded by terminating the OSRD S-l Section and replacing it with the OSRD S-l Executive Committee, consisting of the following: J. B. Conant, chairman, L. J. Briggs, A. H. Compton, E. O. Lawrence, E. V. Murphree, H. C. Urey. H. T. Wensel and I. Stewart were selected to sit with the Committee as technical aide and secretary respectively.
On June 13, 1942, Bush and Conant sent to Vice-President Henry A. Wallace, Secretary of War Henry L. Stimson, and Chief of Staff General George Catlett Marshall a report recommending detailed plans for the expansion and continuation of the atomic-bomb program.
On June 17, 1942, the report was sent by Bush to the President, who also approved its conclusions.
The report contained four principal parts:
Firstly, it was clear that an amount of uranium-235 or plutonium comprising a number of kilograms would be explosive, that such an explosion would be equivalent to several thousand tons of TNT, and that such an explosion could be caused to occur at the desired instant.
Secondly, it was clear that there were four methods of preparing the fissionable material and that all of these methods appeared feasible; but it was not possible to state definitely that any given one of these is superior to the others.
Thirdly, it was clear that production plants of considerable size could be designed and built.
Fourthly, it seemed likely that, granted adequate funds and priorities, full-scale plant operation could be started soon enough to be of military significance.
It was also noted that if four separate methods all appeared to a highly competent scientific group to be capable of successful application, then it was certain that the same desired end result could also be attained by the enemy, provided he had sufficient time.
Therefore it was unsafe at that time, in view of the pioneering nature of the entire effort, to concentrate on only one means of obtaining the result.
In the meantime, however, isotope separation studies at Columbia quickly confronted serious engineering difficulties. Not only were the specifications for the centrifuge demanding, but, depending upon rotor size, it was estimated that it would require tens of thousands of centrifuges to produce enough uranium-235 to be of value.
Gaseous-diffusion immediately ran into trouble as well. Fabrication of an effective barrier to separate the uranium isotopes seemed so difficult as to relegate gaseous-diffusion to a lower priority (the barrier had to be a corrosion-resistant membrane containing millions of submicroscopic holes per square inch).
Both separation methods demanded the design and construction of new technologies and required that parts, many of them never before produced, be fitted to tolerances not previously imposed on American industry.
In Chicago, Compton decided to combine all pile research by stages. Initially he funded Fermi’s pile at Columbia and the theoretical work of Eugene Wigner at Princeton and J. Robert Oppenheimer at Berkeley. He appointed Szilárd head of materials acquisition and arranged for Seaborg to move his plutonium work from Berkeley to Chicago in April 1942. Compton secured space wherever he could find it, including a racket court under the west grandstand at Stagg Field, where Samuel K. Allison began building a graphite and uranium pile.
Although it was recognised that heavy-water would provide a moderator superior to graphite, the only available supply was a small amount that the British had smuggled out of France. In a decision typical of the new climate of urgency, Compton decided to forge ahead with graphite, a decision made easier by Fermi’s increasingly satisfactory results at Columbia and Allison’s even better results in Chicago. In light of recent calculations that cast doubt on the MAUD report’s negative assessment of plutonium production, Compton hoped that Allison’s pile would provide plutonium that could be used as material for a weapon.
Enrico Fermi (1901-1954) won the Nobel Prize in Physics in 1938 for his work on induced radioactivity, and he was the father of world’s first nuclear reactor, the Chicago Pile-1 (CP-1).
By May 1942 Bush decided that production planning could wait no longer, and he instructed Conant to meet with the S-1 section leaders and make recommendations on all approaches to the bomb, regardless of cost.
Analysing the status of the four methods of isotope separation then under consideration, i.e. gaseous-diffusion, centrifuge, electro-magnetic, and pile, the committee decided on May 23, 1942 to recommend that all be pushed as fast as possible. This decision reflected the inability of the committee to distinguish a clear front-runner and its consequent unwillingness to abandon any method. With funds readily available and the outcome of the war conceivably hanging in the balance, the S-1 leadership recommended that all four methods proceed to the pilot plant stage and to full production planning.
With five methods of producing fissionable material, three isotope separation processes (electro-magnetic, gaseous-diffusion and centrifuge) for uranium-235, and two piles (uranium-graphite and uranium-heavy water) for plutonium, Conant called it a “five horse race”. By February 1942, 10 contracts had been made with 12 institutions for a total of more than $1 million, and that figure was doubled in March 1942. In fact they had already decided to allocate between $10 and $17 million for all five “horses” through to early 1943.
In an afternoon meeting on May 23, 1942 the following decisions were made:-
Construction of a 100 gm/day centrifuge plant by January 1944 (cost $38 million)
Construction of a gaseous-diffusion pilot plant (cost $2 million, plus…)
Construction of a 100 gm/day electro-magnetic plant by September 1943 (cost $ 12 million)
One or more piles to produce element-94 by January 1944 (cost $25 million)
Heavy-water production plant for 450 kg/month by May 1943 (cost $2.8 million).
Result: a few atomic bombs for July 1, 1944, and twice as many each following year.
The Manhattan District
On June 18, 1942, Colonel James Creel Marshall, Corps of Engineers, was instructed by the Chief of Engineers to form a new district in the Corps of Engineers to carry out “special work” (atomic bombs) assigned to it. This district was designated the Manhattan District and was officially established on August 13, 1942. The work with which it was concerned was labeled, for security reasons, the "DSM Project" (Development of Substitute Materials).
Marshall was told to form a new engineering district “for construction of a new manufacturing plant”, as part of a project already in progress to develop atomic energy for military purposes. In fact both the U.S. Army and Navy had been briefed by Sachs on October 12, 1939, but ironically, the technical service that eventually had the most to do with the development of the atomic bomb, the Corps of Engineers, was not consulted. In the meeting in 1939 the Army was not enthusiastic, and was even negative about the military potential of such a development. Colonel Marshall was reassigned (August 1943) as commanding officer of the Engineer Training Center, and was promoted to Brigadier General (he later served in Australia, New Guinea and the Philippines). Another report was more blunt and simply said that following frustrating project delays, General George C. Marshall and Lieutenant General Brehon Burke Somervell should replaced Colonel Marshall with a more dynamic officer, and Colonel Leslie Groves took command on September 17, 1942.
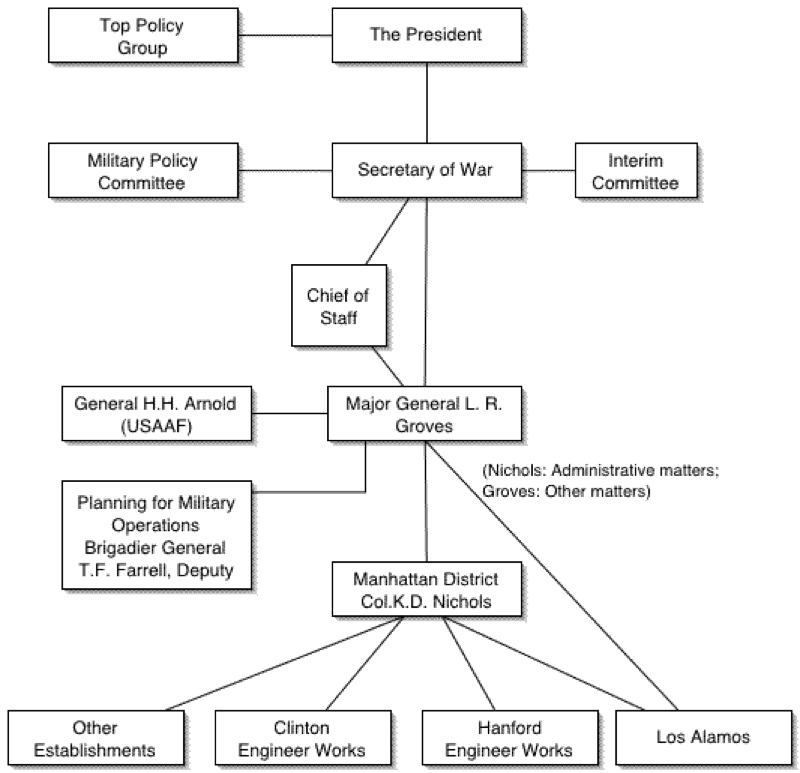
Bush transferred the responsibility for process development, materials procurement, engineering design, and site selection to the Corps of Engineers, and earmarked approximately sixty percent of the proposed 1943 budget, or $54 million.
An Army officer would be in overall command of the entire project. This new arrangement changed the U.S. atomic bomb effort from one dominated by research scientists to one run by the U.S. Army (despite the fact that they knew nothing about nuclear physics).
At the time Marshall was directly subordinate to the Engineers chief, Brig. Gen. Thomas M. Robins, and worked with his deputy Col. Leslie R. Groves. It was Marshall who recruited the core team and set up the liaison office in Washington, D.C. It was Marshall who suggesting using “Development of Substitute Materials” (DSM) as a name, but finally, with Groves, they decided on calling it “Manhattan” (normally Engineer districts took their names from the city were they were located). Manhattan, in New York, was where Marshall had established his temporary headquarters.
It has been written that at this time S-1 and the Manhattan District were free to act on any mutually agreed decision. Site selection became important in their meeting of June 25, 1942. They wanted to pick one site for all industrial-scale construction, at least 200 miles from national borders, with 150,000 kilowatts of electricity available, and hundreds of thousands of gallons of water per minute also available. In addition it should allow construction in winter, have access to a ready supply of labour, good transport access, and have a terrain cut up by ridges to limit the effects of any accidental explosion. A site near Knoxville, Tennessee had been already suggested, the so-called Tennessee Valley (115,000 acres for $3.5 million). At about this time they also selected a site in the Argonne Forest (which I presume became the site for the Argonne National Laboratory in Lemont, near Chicago). And about the same time it was decided that M. W. Kellogg Company would also work on building the diffusion plant. They in fact created a wholly-owned subsidiary, the Kellex Corporation, and a subsidiary of Union Carbide was contracted to operate the diffusion plant.
Objectives, set in June 1943, were that by January 1944 they would have a full-scale centrifuge plant producing about 1 kilogram of enriched uranium-235 per day. The diffusion plant should be a 1-kilogram-per-day production facility, and the electro-magnetic process should be producing 100-grams-per-day. They also wanted a plutonium plant producing 100-grams-per-day, with a heavy-water plant producing 0.5 tons per month.
There were major problems to overcome, despite feasibility being demonstrated. For example, in the diffusion process the barrier needed to be porous to uranium hexafluoride through thousands of stages, and resist the exceptional corrosiveness of the gas.
It was just after the meeting of June, 27, 1942 that it was decided that Stone and Webster would be the managing contractor for the atomic energy project. They would oversee and subcontract all the R&D, procurement, engineering, and construction.
In some of the reports there is a clear reference to confusion. The Executive Committee was responsible for pilot plant studies, and the Army for engineering development and plant design. But in practice it was impossible to separate these tasks. Yet the OSRD and the Army would negotiate separate contracts with different companies for related work on each of the projects. An no one in the Army sat on the Planning board, and no one in the Planning Board had any experience in the Army. And of course the Army had no technical expertise in the field of nuclear physics and chemistry. And finally there was no 'higher responsibility' to resolve differences promptly. Between the two groups there was no ways to handle things that were outside their respective responsibilities, nor was there a way to handle 'hot potatoes' being passed back and forth. Finally those who did have responsibility were slow to use it.
There was one example that perhaps characterised these difficulties more than anything else. There was a need to pick a site for building the first isotope separation plant. The Planning Board got a series of options from the War Production Board and found the idea spot just below Elza on the Clinch River near Knoxville. Wilhelm Delp Styer of the U.S. Army Services of Supply was ready to act, but Colonel Marshall asked for a detailed study of the site. He visited the site, then asked for detailed requirements, etc. But then Colonel Marshall convinced himself that there might not be a need for any site whatsoever. He decided not to proceed with the site purchase until the isotope separation process was proven. The program lost several months. Of course set against this was the total war effort of the U.S. By spring 1942 the government had awarded contracts worth $100 billion, more than the total annual production of the U.S. economy. They had ordered 60,000 planes, 45,000 tanks, 20,000 anti-aircraft guns, and 8 million tons of shipping just for the year 1943.
Another problem was deciding the priority of the Manhattan District. Major military programs received a rating AA-1 to AA-4, with highest being A-1-a (AAA existed for emergencies). The Manhattan District obtained a rating of AA-3 (AA-1 and AA-2 were usually limited for airplanes, ships, guns, etc.), with an optional AAA to “pry loose certain critical items”. This did cause problems, since steel became virtually impossible to obtain without a AA-2 priority. Without a higher priority the atomic bomb program became “an unimportant miscellaneous type”. The District did finally get a blank check to assign the AAA priority as needed, and was also allocated a new priority AA-2X for urgent foreign and domestic industrial programs. In 1943 the District was using more AAA priorities than the total for all Army and non-Army programs combined. The project finally was allocated a full AA-1 priority on July 1, 1944.
You can immediately understand the above issues through a simple example. The problem of the purity of graphite for the pile was always in the background, but once the decisions were made it became a priority. The Metallurgical Laboratory finally found a supply willing to provide tons of very pure graphite, and an order was made with a priority of A-1, for something that had never before been rated above C.
On September 17, 1942, the Secretary of War placed Brigadier General L. R. Groves of the Corps of Engineers in complete charge of all Army activities relating to the DSM Project.
At the time of his nomination Groves was a Colonel, he was later promoted to Brigadier General (in fact his nomination was held back until he obtained his promotion). Groves was an engineer who had been involved in building the Pentagon. Groves had already been advising the District Engineer Marshall concerning power resources and site selection for the Manhattan District facilities.
Groves acted immediately, buying a site in Tennessee, and moving the Manhattan Engineering District to Washington. He also imposed on the scientists a need to select, by late 1942, the best isotope separation technologies for full-scale commitment.
Berkeley, with Lawrence and the electro-magnetic technique, remained the frontrunner. Lawrence refined his 184-inch magnet and huge cyclotron to produce calutrons. The 184-inch cyclotron magnet (see below) was nearly five times wider that the 37-inch magnet used in Lawrence’s previous experiments, and had been totally funded by the Rockefeller Foundation. The recommendation was now to build a pilot plant and full-scale plant on the Tennessee site.

The centrifuge was the big loser. Westinghouse had found it just too difficult to stop severe vibrations during trial runs.
Gaseous-diffusion remained alive, but the problem was still the barrier.
The pile at Stagg Field looked promising, but there was a constant shortage of graphite and uranium. On the other hand Seaborg had developed a way to separate plutonium from irradiated uranium using plutonium’s different oxidation states. In August 1942 he had produced a microscopic sample of pure plutonium. It was even decided to build a production facility at Site X at Clinch River in Tennessee. Groves wanted Du Pont to take on this challenge, but they felt that the mass production of plutonium could only be achieved by 1945.
Glenn Theodore Seaborg (1912-1999) won the Nobel Prize for Chemistry in 1951 for his discovery of ten transuranium elements (including the actinides).
Site X was the original name given to the 59,000 acres of land along the Clinch River, it was only after the WW II that the name was changed to Oak Ridge.
On August 4, 1944, an incident occurred with the plutonium chemist Don Mastick at Los Alamos. Liquid plutonium in a vial had undergone an unanticipated transformation overnight. Some of the liquid became a gas and was ingested by Mastick. At the time, the medical doctor at Los Alamos was Louis Hempelmann. He was only 29 years old, and like Manhattan’s Dr. Alderman, he had little understanding of the effect of radiation on the human body. After hearing Mastick’s account, Hempelmann made a frantic call to Stafford Leak Warren, the medical director of the Manhattan Project (and inventor of the mammogram). Warren was an Army colonel who worked at the project’s administrative headquarters in Oak Ridge, TN, and who was responsible for conducting research on the health effects of radiation exposure (and reported directly to General Groves). Warren told Hempelmann to use a mouthwash, expectorant and stomach pump to remove the plutonium. So Mastick was handed a four-litre beaker of “murky liquid”, and then, as the resident plutonium chemist, Mastick was later responsible for retrieving the plutonium from his own fluids.
The reality is that the ingestion of plutonium does not cause adverse health effects. Manhattan Project chemist Lawrence Bartell had a similar experience while performing an experiment with plutonium at the University of Chicago. “One of the things that we had to do was to pipette solutions to get the right amount to mix and to precipitate”, recalls Bartell, and “one day the pipette backed up and I found myself with plutonium in my mouth”. Bartell, who was embarrassed by his mistake and was too ashamed to go to the health physics division, rinsed out his mouth as best he could and went about his day. According to Bartell, plutonium “goes through the body fairly fast if taken orally”. If it gets into your lungs, however, “you’re in very bad trouble”.
Plutonium is toxic, both chemically and because of its ionising radiation, but there are many substances that are more toxic (kg for kg), such as arsenic, cyanide, etc. If you were to eat (ingest) plutonium it would pass through the gastro-intestinal tract and be expelled without causing much harm. The main danger comes from inhalation, particularly with particles sizes less than 0.01 mm. Most particles would be exhaled or expelled by the mucous flow from the bronchial system into the gastro-intestinal tract. But some particles would be trapped and find their way through the lungs into the blood or lymph system, and to the bones or liver. These deposited plutonium particles are alpha emitters and could cause cancer.
In the late 1930's, Du Pont had introducing nylon, the first synthetic polymer. Behind this success was a new technique for continuous operations with the ingredients at one end and the product at the other. The process was revolutionary, a vast improvement over earlier step-by-step approaches. Groves was convinced that Du Pont could apply the same ingenuity to the plutonium production process at Hanford and tried to persuade Du Pont to join the Project at the end of October 1942. Despite Groves' urging, it took a call from President Roosevelt to convince Du Pont to accept the task.
In fall 1942 Robert Oppenheimer, leading a group of theoretical physicists, suggested that the critical mass needed would be about twice that initially expected. However they also reported that fusion explosions using deuterium (heavy hydrogen) might be possible. The possibility of thermonuclear (fusion) bombs generated some optimism since deuterium supplies, while not abundant, were certainly larger and more easily supplemented than those of uranium and plutonium. Basic research on other light elements was authorised.
In December 1942, Roosevelt gave final approval to construct a nuclear bomb.
So the centrifuge project was cancelled, and gaseous-diffusion (lower priority), the pile, and electro-magnetic separation were to proceed to full-scale development, eliminating the pilot project stage. In November 1942 the Lewis Committee elevated gaseous-diffusion to first priority.
World’s First Self-Sustaining Chain Reaction
At 3:20 p.m. on December 2, 1942, Fermi’s massive lattice pile of 400 tons of graphite, six tons of uranium metal, and fifty tons of uranium oxide achieved the first self-sustaining chain reaction, operating initially at a power level of one-half watt (increased to 200 watts ten days later).

This is the only original photograph of the reactor being built.
As Compton reported to Conant, “the Italian navigator has just landed in the new world”. To Conant’s question, “Were the natives friendly?” Compton answered, “Everyone landed safe and happy”. Significant as this moment was in the history of physics, it had already been decided to move to the pilot plant and Groves had already instructed Du Pont to move into design and construction on December 1, 1942.
For the test, most people were on the balcony of the court. Norman Hilberry, equipped with an axe, was prepared to sever a rope tied to a balcony rail. This would drop into place an emergency safety rod suspended over the pile (Hilberry went on to direct the Argonne National Laboratory). George Leon Weil handled the final control rod. On the platform above the pile, three men stood ready to flood it with cadmium salt solution, which would absorb sufficient neutrons to halt a runaway reaction (they were called 'the suicide squad'). Also in the control room they had command of the safety rods. As the last control rod was removed, foot-by-foot, tests were performed. Halfway through Fermi said he was hungry, and they broke for a lunch lasting a little more than two and one-half hours. They continued removing the control rod shortly after 14:00, and declared that the reaction was self-sustaining at 15.25. Wine from a now famous flask of Chianti was poured into a paper cup and passed around. No toast, no remarks, nothing.
On December 28, 1942, Roosevelt approved an investment of $500 million to build full-scale gaseous-diffusion and plutonium plants and the compromise electro-magnetic plant (smaller than the others), as well as heavy-water production facilities.
The procurement and engineering functions of the Planning Board were taken over by the Manhattan District in the summer of 1942 and by the spring of 1943 the Manhattan District took over the research and development contracts from the OSRD. The transfer was made on May 1, 1943, and marked the end of the formal connection of OSRD with the uranium project.
In many way the Manhattan Engineering District had become a big construction project. It purchased and prepared sites, let contracts, hired personnel and subcontractors, built and maintained housing and service facilities, placed orders for materials, developed administrative and accounting procedures, and established communications networks.
By the end of the war Groves and his staff had spent approximately $2.2 billion on production facilities and towns built in the states of Tennessee, Washington, and New Mexico, as well as on research in university laboratories from Columbia to Berkeley.
What made the Manhattan Project unlike other companies performing similar functions was that, because of the necessity of moving quickly, it invested hundreds of millions of dollars in unproven and hitherto unknown processes and did so entirely in secret. Inherent to this approach was what is now often called trial-and-learning experimentation, a way to mix problem solving and innovation. Also called 'experimentation in the unknown', the focus was (and still is today for many companies) on inventing something while dealing with incomplete knowledge or even profound ignorance. This type of experimentation is often used when there are several different problems to solve, when there are many alternatives to test, and when the evaluation criteria are not pre-given. When the Manhattan Project started the fundamental principles were clear, but theories were full of unverified assumptions. In some cases no theories were available, so experimentation (trial and error) was the only solution. Groves called it “proceeding in the dark”. For example, the work on electro-magnetic separation was all about magnetic shims, sources, and collectors that gave the best results, although no one could explain why one combination was better than another. Perhaps the most obvious example was the development of the implosion technique. The starting point, in July 1943, were incredibly crude experiments using TNT surrounding hollow steel cylinders. It was John Von Neumann, in September 1943, that suggested arranging shaped charges in a spherical configuration around the active material, and aim for a faster kind of implosion based upon increasing the amount of high explosive. The final assembly consisted of a 5-foot diameter sphere composed of 'lens' for a total of nearly 3 tons of high explosive. The theory work only started in January 1944, with the analysis of Edward Teller on the (shock wave) hydrodynamics of implosions. Calculations were impossible by hand, and were at the limit of the computing power available at that time. This was one of the reasons why Los Alamos became a pioneer in using computers (they received a big IBM machine in April 1944). In 1944 there were analytic solutions for shock waves in perfect gases, but nothing for nuclear materials in a temperature-pressure-energy region of interest in an atomic bomb. Despite that, Teller was able to show that an implosion was possible, and he was able to define the kinds of experiments needed. The impact of these initial calculations and experiments was that two new divisions, 'Gadget' G Division and Explosives X Division, were created. Throughout the first half of 1944 the focus was on how to measure (extremely fast) implosion parameters such as symmetry, time of collapse, and degree of compression. They ended up identifying seven different approaches, ranging from X-ray photography, through the RaLa method, to a so-called betatron method. For example, the X-ray photography revealed 'jet' formations during implosion, and at the same time pushed the development of very precise timing circuits. In this particular example, the RaLa method, invented by Robert Serber in November 1943, involved placing a gamma-ray source in the centre of the spherical assembly. The gamma-rays would travel out radially, and where affected by the density changes in the collapsing sphere of metal. The data provided time of collapse, and degree of symmetry and compression. Nice idea, but they then had to find a good gamma-source (so-called RadioLantanum-140) and design new ionisation chambers (that would be destroyed in the explosion), new recording systems, and build a test site with a bomb-proof shelter. Just getting RadioLantanum-140 required Oak Ridge to build a special extraction laboratory and a second plant for dissolving irradiated uranium slugs and recovering barium from the solution (and then secretly ship it 1,200 miles to Los Alamos). RadioLantanum-140 was the most powerful radiation source people had worked with at that time. Experiments started September 22, 1944, and only on December 14, 1944 did it really work properly. Electronic detonators appeared in February 1945, along with the first observation of sizeable compression. And by June 1945, they had a viable design for the explosive 'lenses'. Some experts have summarised this entire process as “blowing things up and looking to see what happened”. There was no established art for implosion devices. Experiments were the only option with no 'a priori' knowledge. And each experiment lead to new questions and new problems, which lead to new multi-disciplinary groups being formed. And each experiment required a new generation of measurement instruments (the experiments were not possible with existing technology). A key (hidden) concept seen in this example, was to tell the difference between failures and mistakes. Mistakes were wrong experiments that did not help in the understanding of a problem, failures were to be learned from and pointed to improvements needed. The reality was that the implosion example had both, so it was important to understand the difference and learn (in different ways) from both. In some cases there was no way to tell the difference between a mistake and a failure, so the key was to “amass a body of understanding” through a series of overlapping experiments, none of which would individually have been able to provide the solution.
One nice 'general' rule that has been identified is that if you are really looking at a problem that requires radical innovation, then the available instruments of measurement and test are almost always inadequate, or even inexistent.
For the Manhattan Project the need for haste clarified priorities and shaped decision making. Unfinished research on three separate, unproven processes had to be used to freeze design plans for production facilities, even though it was recognised that later findings inevitably would dictate changes. The pilot plant stage was eliminated entirely, violating all manufacturing practices and leading to intermittent shutdowns and endless troubleshooting during trial runs in the production facilities.
In July 1943 Conant and Richard Chace Tolman were formally asked by General Groves to serve as his scientific advisers. They had already been doing so informally and continued to do so. Coordination of the various scientific and technical programs was accomplished by meetings between General Groves and the leaders of the various projects, in particular, Compton, Lawrence, Oppenheimer, and Urey.
The Physics of the Pile
You are going to have to still live with a multitude of terms describing the same thing, e.g. pile, core, and reactor all mean the same thing, and uranium usually meant natural uranium oxide, and metal often meant uranium metal, etc. And of course the wonderfully evocative 'lumps' was just a term used to describe the uranium blocks or units that were the fuel for the pile.
We are going to try to delve into the physics of pile as it evolved. We will start with what looks to be a really secondary problem. In early 1942 the question arose, if a pile generated heat, would it be more reactive or less? For safety reasons it would be better that the pile had a negative temperature coefficient (for a short period the Chernobyl reactor had a positive temperature coefficient and we all know what that meant). In simple terms a negative temperature coefficient means that if the pile's operating temperature is exceeded the reactivity is reduced. In fact the temperature coefficient is one of the most important physical quantities that defines the inherent stability of a reactor. Important, but difficult to calculate, and so it is often something that is measured in critical assemblies (such as the Chicago pile).
What can happen when the temperature in a reactor increases? Firstly, the resonance absorption increases due to the broadening of the resonance levels. Secondly, the fraction of thermal neutrons lost by absorption in the moderator decreases. Thirdly, the diffusion length of thermal neutrons increases, and so does loss through leakage outside the pile. This third factor is dependent upon the physical geometry of the pile.
Femi concluded that for a critical pile, an increase in temperature of 700°C would produce a small increase in the multiplication factor, nevertheless when the first pile was built the temperature coefficient was one of the first things Fermi wanted to measure.
As we continue to follow Fermi, we have to move to Chicago, and we will see the idea of a large-scale pile experiment become a reality.
In Chicago one of Fermi's 'new' collaborations was with Gregory Breit on the topic of reflectors and what they called a 'seed' or core. The starting point was that a natural uranium and graphite system was not likely to have a very high multiplication factor ƙ, more than 1.0, but not high. Things were expected to be better with uranium metal and a purer form of graphite. One idea was to concentrate the higher quality graphite in the 'core' and surround the core with some other 'neutron regenerative structure' having a lower multiplication factor. It turned out that using a reflector reduced considerably the amount of enriched uranium needed. In addition it was found that there would be more neutrons in the reflector than in the core or 'seed', so that the main production of plutonium-239 could take place in the reflector.
The volume needed for a chain reaction with a seed but without a reflector was about 180 tons, consisting of about 32 tons of natural uranium oxide. With a seed and a reflector the amount of natural uranium oxide needed was reduced by between ⅛ and ⅓ of the amount needed without a reflector, their best estimate was a requirement of 11-12 tons instead of 32 tons.
As Fermi waited for the arrival of sufficient material for his first chain reaction, he continued to improve his understanding of a variety of physical constants. His interests ranged over absorption cross-sections for thermal neutrons in both uranium oxide and graphite, the effect on neutron leakage due to the presence of many empty ducts through the pile, the effect of temperature changes on the multiplication factor ƙ, and the nuclear properties of various materials likely to appear in a industrial pile.
Also during this time the exponential pile was constantly being built, modified, and re-built, depending upon the latest experiments. One particular series of tests showed convincingly that the latest delivery of 'pure' uranium oxide was truly chemically pure, and that the uranium oxide used a year ago in Columbia had been "filthy". Fermi found a multiplication factor ƙ of 1.04 for exponential pile No. 9 (i.e. the 9th re-build of the same basic components).
In Chicago Fermi repeated an experiment he had conducted in Columbia, the measurement of the number of neutrons emitted by uranium per thermal neutron absorbed 𝜼. In fact in Chicago Fermi could direct a team to perform all the experiments he wanted, and even have the results analysed by team of theoreticians. In this case he did the analysis himself. The issue was to use an experimental set-up with a better geometry and with purer materials. The results Fermi obtained was 𝜼 = 1.29, which was significantly lower than the figure he had found at Columbia. All the more reason to check and re-check everything.
According to Fermi's notes the site just outside Chicago was originally bought in June 1942 to house the future reactors for producing element-94 (plutonium). The Army quickly realised that the site was not big enough, so the Oak Ridge site was purchased. The so-called 'Argonne Forest' site was freed for everything but the construction of the first production reactors. So it was there that the team decided to build the experimental pile. Eventually the plans would change again and the pile would be built on the university campus.
In September 1942 the weekly reports started to include a section for the preparatory work for the experimental pile. The first experiment that I've come across was about the idea to enclose the pile in a rubberised balloon cloth which would allow the pile to be evacuated of air (in the end the core was not evacuated). Tests were also done on cooling the granite pile with water running through copper pipes embedded in the outer portion of the graphite. They also tested the idea to fill the evacuated balloon cloth with helium. In each case they studied the energy dissipation per unit area under 'operational' conditions. Work also progressed on the physics of the four control rods.
From October 1942 the focus on the experimental pile increased (still planned to be built in the Argonne Forest). It all started by pressing about 50 tons of uranium oxide and machining about 500 tons of graphite. This work was done sitting under the 'famous' West Stand. Over time the general idea of the pile evolved. In October it had become a spherical structure of about 9 m in diameter with a central lattice holding lumps of about 2 kg each. In parallel work continued with exponential experiments with uranium metal lumps.
What we see above is a lattice of lumps, partly of uranium oxide and partly uranium metal embedded in graphite. The entire pile was made up of cubes of 21 cm on a side. About 6 tons of metal was available so the idea was to build a pile of approximately spherical shape using the best graphite and uranium metal in the centre. The pressed uranium oxide lumps weighted about 2.1 kg each. Finally it did not work out as a real sphere, but it was 309 cm top-to-bottom and 388 cm across the equator. There was a wooden structure that kept it all together. The calculations for this design put the multiplication factor ƙ at 1.039.
More or less in parallel with the work on the Chicago pile Fermi chaired another team looked at how to build a reactor for producing plutonium. One of the first issues was the choice of a cooling system with a sufficiently low neutron absorption. There were lots of ideas, ranging from liquid bismuth, through liquid uranium hexafluoride, to uranium oxide as a liquid slurry. It was clear that to produce plutonium the reactor(s) would need to dissipate 100's of megawatts of heat. And corrosion and radiation shielding would also be additional problems. The real challenge was that the best engineers had never even heard of a neutron!
Naturally Fermi also studied some of his own ideas for cooling. The first was with a system of water pipes passing through the graphite structure and the core of uranium fuel. The heat from the lumps must be conducted through the graphite to the water tubes. The other alternative was to put the uranium metal in cylinders (rods) packed together and running through the graphite, and then to run a single water tube though the middle of these cylinders. In some ways one was cooling the graphite and the other cooling the fuel. Fermi weighted up the pro's and con's of the two options. The water pipes fitted in with the way the core design had been modelled, so the neutron properties were well known. The small number of cooling pipes would have a limited impact of the reactivity of the pile. The down side was that the clearances between the graphite, pipes and lumps must be small, and there may be problems with thermal distortion of the pile. Also it would be difficult to disassemble the pile after the tests. Concerning the second idea it would be easy to disassemble the core, and the fuel would be easy to handle and reprocess. The construction would be easy, and no particular precision would be needed. The down-side was the large loss in reactivity, and the uranium would need to be prepared in 'shots' (what we would call pellets today). Fermi's conclusion was that both ideas were not perfect, and perhaps surface cooling of the pile was the better idea. This might mean running the reactors at a lower output, but might make the reactors easier to operate.
In November 1942 most the basic material for the pile had arrived, and the task at hand was to start building the pile. But there was a strike of the labour unions, so Fermi simply proposed to build the pile in the West Stand, even if it would have to run at nominal power. Compton gave Fermi the go-ahead, and they started building on November 14, 1942.
It's impressive to note that on the one hand Fermi was starting to plan the first test of the pile, and on the November 18, 1942 Du Pont had accepted to build large scale piles for plutonium production at the Hanford site.
Whilst the decision to build the pile in the West Stand dates from November 14, 1942, in fact Fermi had been building the pile there from early October. As the graphite arrived the bars were machined to size and holes were drilled to accommodate the uranium lumps. The machines used were normal wood-working machines, and some of the bricks were drilled with a 8.35 cm hole to take the uranium spheres (which they called 'briquettes'). A second team was pressing the uranium oxide powder into spheres using a large hydraulic press (pseudo-spheres of 8.35 cm diameter). The press used had a force of 150 to 175 tons. By October 15, 1942 210 tons of graphite had been machined, and on November 16, 1942 they opened the rubberised balloon cloth envelop and started to build the pile. Work continued 24/7 by shifts. The pile itself was built within a wooden frame, and each day they segregated the better quality graphite from the lower quality. Fermi would calculate each day where to put the different grades of graphite (remember no computers, everything was calculated by hand). As they built the pile layer by layer they had also to decide where to put the uranium oxide and the uranium metal. An to top it all, more uranium metal arrived which made them adjust the core configuration on-the-fly. Then there was the problem of the location of the cadmium control strips. These control 'rods' were in fact just cadmium sheets nailed to a flat wooden strip to be inserted in a slot machined into the graphite. They made sure the pile remained sub-critical, the control rods were inserted as the pile was constructed, and locked in place with a padlock. One additional control rod was made to drop in by gravity, it was called the 'zip'. The idea was that it would be suspended by a cord over the pile, a cord that could be cut in an emergency.
In all 2,060 uranium metal cylinders (5.7 cm long) were made, 14,840 uranium dioxide spheres, 1,200 triuranium octoxide spheres, 540 uranium dioxide cylinders and 840 triuranium octoxide cylinders, for a total of 46.5 tons. When they talked of spheres or pseudo-spheres what that meant was a cylinder of equal diameter and height but with the edges cut off to make them roughly spherical.
As the layers of the core were built up, reactivity measurements were made for each layer. They were able to predict that the pile would go critical on the 57th layer, during the night of December 1, 1942. During that evening the last measurement was made, and it was clear that with the removal of the last cadmium rod the pile would go critical.
The next morning the last cadmium rod was pulled step by step. The neutron activity was measured, and compared with the previous measurement and with the predicted value. Everything went according to plan, and with the complete removal of the last cadmium rod the pile went critical, and the worlds first self-sustaining chain reaction took place. Some 40-odd people were present, including Anderson, Compton, Fermi, Szilárd, Wigner,…., and also Crawford Hallock Greenewalt of Du Pont, who wanted to know that what they had contracted to build actually worked. The pile worked for 28 minutes, with an energy production of less than a watt.
Above we have the automatic intensity recording for the first operation of the pile. We can see the exponential rise of the intensity. What we see is neutron intensity increasing from bottom to top, and time along the horizontal axis. The first thing we see on the left is the removal of the control rods. Then you see the levelling of the neutron intensity indicating that the pile is not yet critical. The big drop at about the 6th interval from the left was simply a change of scale. On the far right you can see a sharp rise to a peak and a sharp drop. This is the signal of the self-sustaining reaction, a signal that shows no sign of levelling off. The sharp drop in the signal at the end was where the control rods were reinserted in the core.
We see mentioned that Greenewalt of Du Pont was present during the Fermi's first demonstration of a self-sustaining chain reaction. In fact the story is far more complex. With the arrival of 'big-industry' with Du Pont (electro-magnetic, pile and isotope separation) and Kellogg (gaseous-diffusion) Groves decided to re-appraise the entire Manhattan Project. He turned to Warren Kendall Lewis to head the review. The Committee visited Columbia for the gaseous-diffusion work. The review was positive in that they felt that the barrier was similar to a catalyst in chemical engineering, and the process looked like something that could be perfected in the chemical industry. On the other hand they did not think that the partnership Kellogg-Columbia could do the job. They then went to Chicago. The Du Pont people had visited the site some 3 week earlier, but for Lewis it was new. They listened to the presentations, but there was nothing to see (Fermi had not yet demonstrate a chain reaction). They then left for Berkeley to look at the electro-magnetic process. They saw the calutrons but remained unconvinced. They then took the train back to Chicago to see the demonstration of Fermi of the first self-sustaining chain reaction. That is why Greenewalt was there, and why he returned to the other member of the Lewis committee with "the glow of success on his face". Even without this demonstration, the Lewis committee had already recommended to continue the work on the pile. They also recommended to build a full-scale gaseous-diffusion plant and to push forward with the calutron (although they felt that a full-sized plant with 22,000 calutrons was not practical). They thought that a pilot with 100 calutron's could produce uranium-235 for use in physical measurements, but they could not see beyond a 100-gram plant.
On the December 12, 1942 the pile was tested again but this time at a power production of about 200 watts for 45 minutes.
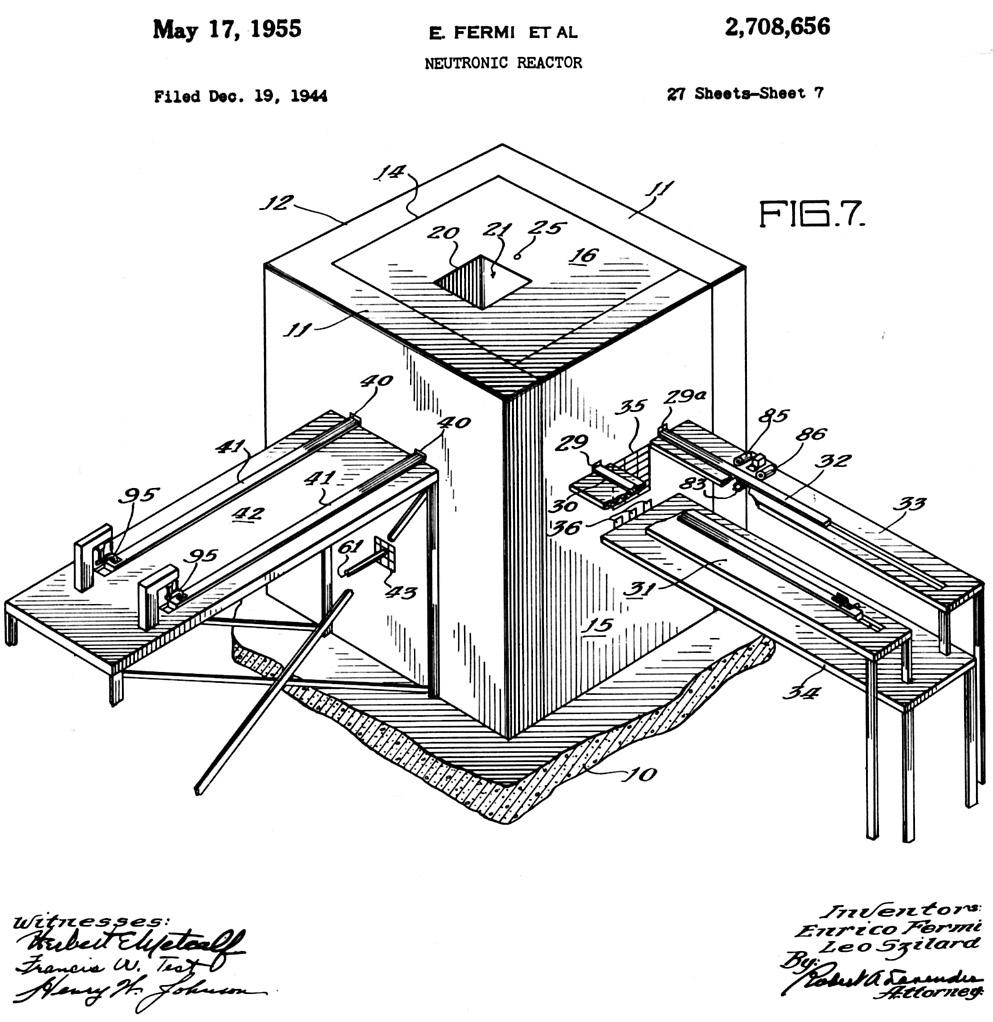
Above we have one of the diagrams from the U.S. patent held by Fermi and Szilàrd for a 'Neutronic Reactor', i.e. the construction and operational of self-sustaining neutron chain reacting systems with the production of power in the form of heat. What we see above is a reactor with a heavy concrete foundation (10) with side walls (11) and connected back wall (12). A vault space (14) is created in which a chain reacting lattice of uranium and graphite is erected until the vast is filled within about 2 meters from the top and from the front. The front is then completed with a concrete wall (15), and top (16) is added made of wood and lead layers. A hole in the top (20) leads to a well (21) extending inwards to the peripheral layer of uranium bodies in the internal lattice. A small shaft (25) leads to the central position of the reactor. The front wall (15) is pierced by 'shim' and regulating rod apertures (29 and 29a). A 'shim' or limiting rod (30) is positioned on a platform (31) and is moveable, and a control rod (32) is positioned in a similar way. Below there is a removable platform (34) that can receive lattice portions that can be removed from the reactor through a channel (35) and from the stringer channels (36). Two safety rods (41) can be inserted through apertures (40). Below there is an ionisation chamber channel (43).
The self-sustaining chain reaction occurs by building up within the space (14) uranium metal and oxide and graphite blocks, creating a 'pile'. Certain graphite blocs have holes for the uranium bodies ('live' graphite), those that do not have holes are 'dead' graphite. The uranium bodies are placed in the holes in the live graphite, and are used to build up the chain reacting system. As the reactor is built layer by layer the ionisation chamber, the shims, the regulating rods and the safety rods are added. The patent describes the experimental operation of the reactor as well as the design of the components, including the fuel, graphite and different rods inserted through the pile.
Fermi's co-author on may papers, H. L. Anderson, produced a wonderful observation on Fermi and on the newly constructed pile. The pile promised atomic energy and for some atomic bombs. But it also promised something new and unexpected. The pile because a fantastic experimental tool. Fermi noted that experiments that were almost impossible to perform before, became easy beyond his wildest dreams. The pile was a kind of neutron multiplier. Very minor changes to the neutron population were multiplied a million times. For example the temperature coefficient of the multiplication factor, long a difficult problem, could now be measured with great precision. The pile was sensitive to some cold air entering the room through an open window. The pile was an excellent device to check the purity of uranium, and for the study of the effects of really minor changes to the lattice design, etc.
Finally the original West Stand pile, named Chicago Pile 1 (CP-1) had a short life, and after three months it was taken apart and replaced by CP-2 built at Argonne Forest. The focus of the work changed, and CP-2 and later piles were important in connection with the design of the Hanford plant (site W). Uranium metal was tested, control rods were designed and tested, shielding was tested, the presence of water in the pile was studied, etc. By October-November 1943 pile engineering had been more or completely transferred to Oak Ridge (some times known as Site X or Clinton Engineering Works). In addition they had a working pile that was producing small quantities of plutonium sufficient to test chemical separation.

The impression given is one of am experiment rapidly thrown together in a dingy corner of an old football stadium. There are no photographs of that moment, but above we can see that CP-3 built at Argonne Forest was far from some 'Lego' set up in a corner somewhere.
By April 1944 more and more people from the Chicago team were working in Hanford, and Fermi himself was increasingly being called to Los Alamos (he moved there in September 1944 but moved back to Chicago December 31, 1945).
Nuclear Physics - Simplified
At one point in time Fermi tried to explain what was planned using 'simple' language. He talked about the physics of the pile, and the meaning of the multiplication factor ƙ (which Fermi always called the reproduction factor). Fermi's definition of ƙ was the "number by which one multiplies the number of neutrons of one generation to find the number of neutrons in the next generation". So if you have Ni neutrons in generation i there will be ƙNi daughter neutrons and there will be ƙ2Ni neutrons in the 2nd generation, and ƙnNi neutrons in the nth generation. If ƙ<1, then ƙnNi will tend to 0, and the reaction is not self-sustaining. If ƙ=1, then the reaction is self-sustaining, and if ƙ>>1, then "run as fast as you can and get behind a big hill miles away".
Fermi went on to explain in a little more detail the life of a neutron. A neutron is born in a uranium lump with a kinetic energy of 2 MeV, and before it escapes the lump it can collide elastically with a uranium atom, transferring a small part of its kinetic energy to the translational motion of the nucleus but leaving the state of the nucleus unchanged. The neutron will travel on with a slight loss in kinetic energy. The neutron can also collide inelastically with a uranium nucleus, it can escape but it imparts a larger part of its kinetic energy to excite the nucleus to a higher energy state. The nucleus may then, for example, emit a gamma-ray. The neutron can also collide with a nucleus and caused fission. In this case the neutron is captured. Fission in uranium-235 can be caused by both fast and slow neutrons. To cause fission in uranium-238 the neutron must have an energy greater than 1 MeV. The chance of this happening for a 2 MeV neutron born deep within the lump is about 15%. In general when fission occurs the incident neutron disappears and a little less than 2.6 neutrons appear, i.e. a multiplication factor of about 1.6 (average multiplication factor per neutron for the process of fast fission in natural uranium metal).
However, most of the neutrons actually escape the uranium metal lump because they are born nearer the surface. This is in part due to the fact that they are created from fissions induced by thermal neutrons which come in from the graphite and die near the surface of the uranium metal lump. Thermal neutrons have a high probability to be absorbed in the surface of the lump.
As neutrons slow they may be caught in the resonance bonds of the metal uranium. This resonance absorption is a function of the volume of the uranium metal lump. If the neutron has the energy corresponding to a resonance absorption peak it is almost immediately absorbed as it hits the lump, and thus the number of neutrons absorbed is proportional to the surface of the lump. If the neutron energy does not correspond to a high absorption coefficient it may travel some distance into the lump before finally being absorbed, so the number of neutron absorbed by a lump is proportional to the mass of the lump.
The way the pile was designed the idea is that about 0.887 of the fast neutrons will escape resonance absorption and will thus reach thermal energies. For every thermal neutron produced, only 0.868 thermal neutrons are re-absorbed by the uranium metal, and for every thermal neutron absorbed, 1.29 neutrons will be produced. From this 0.887 x 0.868 x 1.29 = 0.993 is the fraction of new neutrons from slow fission per original fast neutron. Correcting for the contribution of fissions by fast neutrons, yields the multiplication factor ƙ, which in theory should be 1.086 (the average number of neutrons produced by one neutron).
What Fermi planned was to create a self-sustaining chain reaction, with the initial neutrons provided by spontaneous fissions in the pile. The energy of these first neutrons will be reduced so that when they finally come into contact with 'special materials' new neutrons are born very readily. A fraction of this new generation of neutrons then repeat the life history of their parents.
The pile will be a big cube of graphite 6.4 metres on each side, and consisting of carbon blocks and uranium units. The uranium will produce neutrons and the carbon blocks will slow them down (or 'moderate' them).
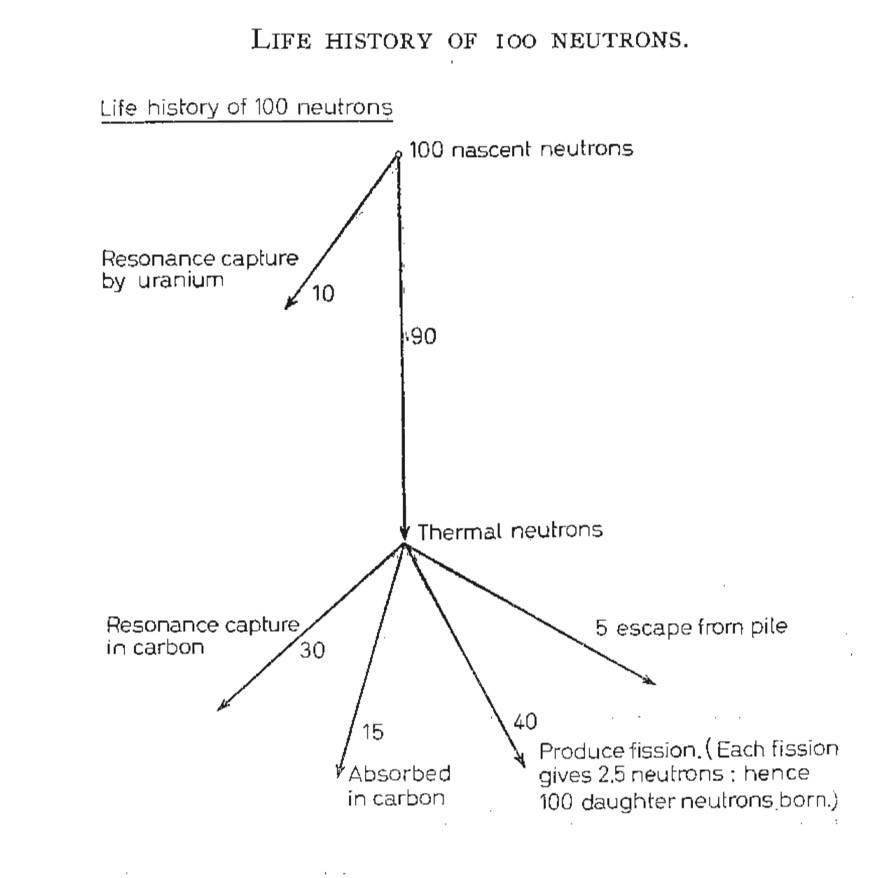
There are three things that can happen to the neutrons (the above diagram comes from one of Fermi's reports). Firstly, neutrons can escape, and to reduce this the pile has been made very big. Secondly, neutrons can be simply captured and disappear. Thirdly, they can produce a fission and release daughter neutrons. The geometry of the pile is designed to reduce the second probability and increase the third.
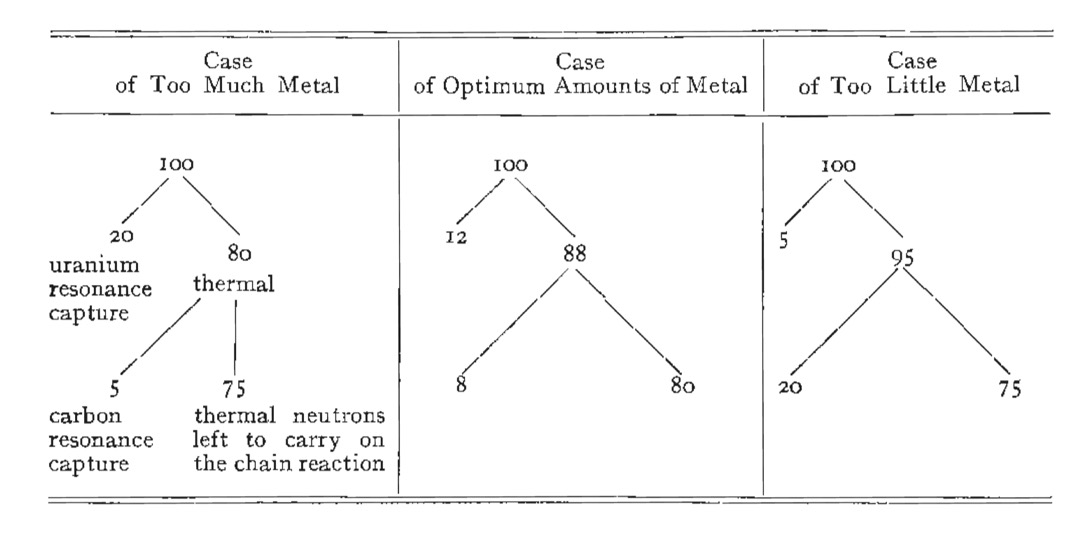
Again taken from one of Fermi's original reports he describes why a particular lattice structure was chosen. When Fermi mentions 'metal' it means uranium metal. The key is to manage the number of neutrons lost by thermal and fast resonance absorption, these are lumped together in the single figures 20, 12, and 5. Some neutrons will die in the uranium and some in the carbon, both will be lost to the chain reaction. It there is too little metal more neutrons will die by uranium resonance absorption, but if the number of neutrons lost by carbon and uranium resonance absorption is minimised more neutrons are left for the chain reaction.
There will be special neutron counters in the pile to measure the total number of neutrons in the pile at any time (neutron density), and ƙ will be these neutrons minus those that manage to escape from the pile. The pile will be built up with 4 rods specially designed to absorb thermal neutrons. These rods can be moved in and out horizontally and control ƙ. When the pile is complete the whole will be in a rubberised balloon cloth and will be evacuated to remove the air, reducing even more any parasitic capture. Then gradually the control robs will be pulled out and ƙ will approach 1. If and when this happens everyone will leave the area and they will try to see if power can be obtained from the pile.
Put like that it sounds simple!
Another task that Fermi took on was to brief the engineers who would build the plutonium piles at Hanford. One masterful contribution was on the idea of a self-sustaining reaction. The whole question turned about two factors. The first was the multiplication factor (reproduction factor) ƙ. A big pile (or reactor) will be 'chain-reacting' if ƙ>1. If ƙ<1 then it does not matter how big the reactor is. Secondly, some neutrons will be lost from a reactor of a finite size, and the key is to retain as many as possible inside the pile. The bigger the reactor the smaller the loss, for an infinite reactor the neutron loss would be 0%. Interestingly if we define something called the coefficient of retention l, then ƙl = 1. If the multiplication factor is greater than 1 by only a very small amount, the size of the chain-reacting system would have to be very large in order to minimise the loss of neutrons.
It would be great to be able to calculate an exact multiplication factor ƙ but it was found to be impossible because it was not practical to measure with precision the numerous constants involved in the equations (in 1942 the error would have been about +/-15%). Calculations pointed to a ƙ of about 1, but would it be above 1, or below 1? So the only way was to measure ƙ as precisely as possible, and the important thing was that different configurations resulted in the same ƙ to within 1%. During the experiments what emerged was that the materials used must be of an exceedingly high purity, particularly free of boron and several rare earth elements. During the experiments a ƙ=1.04 was found, and a ƙ=1.07 was predicted for uranium metal. The experiments were not full sized piles, so there remained the question about the precise amount of materials that would be required for a chain-reacting system. Knowing ƙ to within 1% just meant that they knew the amount of materials needed to +/-40%. For a metal-graphite system the error would still have been +/-15%.
Another problem with a full-sized system was how the reactivity of the system might vary with temperature. If the temperature rose with a rise in reactivity, then an accidental increase in temperature could have be very dangerous. If the reactivity decreased with increasing temperature, then the system would be thermally stable. Even in 1942 the tests performed showed that this second situation was the most likely, but the small changes they were measuring were about the same as the experimental error! At the time of the experiment on a full-size pile this question was still outstanding. It was for this reason that a controlling mechanism was needed so that the reaction could be interrupted.
The thermal stability of the system was not guaranteed, therefore a control system was installed. The key was to use a material that had strong neutron absorption characteristics. The idea was to have solid rods that could be pushed mechanically in to slots in the pile. An alternative might have been to use a liquid that would fill up pipes inside the pile. A gas might also have done this if it were introduced under pressure and filled all the empty spaces. The option of solid rods was retained, but it was predicted that in a power producing reactor they would also need to be cooled to avoid warping of the rods.
In addition to the control rods, a separate set of rods should be kept as safety rods. These rods should be designed to work in all conditions, including should they warp. Fermi felt that an additional back-up might be needed, and he suggested the possibility to flood the system with a neutron absorbing liquid.
As an extension of this section on 'simplified nuclear physics' I have added below the description used by Fermi and Szilàrd in their U.S. patent for a 'Nuctronic Reactor'. It describes the chain reaction process, as it was known at the time. What the authors described was a finite system using natural uranium bodies or lumps dispersed in a graphite moderator within a larger reactor body where the neutron density is substantially constant. I am using the language and terminology from the patent file.
A represents fast neutrons being set free as a result of a fission process.
B represents a fast neutron loss due to leakage out of the system.
C represents a uranium body of any size in which both volume and surface resonance absorption of neutrons by uranium-238 takes place, at resonance energies above the thermal energy, and leading to the formation of element-94.
D represents the number of neutrons reaching thermal energy.
E represents a thermal neutron loss by diffusion out of the system.
F represents a neutron loss caused by capture of neutrons by impurities in uranium, graphite, and controls.
G represents a neutron loss due to capture of thermal neutrons by the graphite as the thermal neutrons diffuse before emerging uranium.
H represents the number of thermal neutrons entering a uranium body.
I represents a uranium body of any size in which part of the thermal neutrons entering the body are absorbed by uranium-238 leading to the formation of plutonium-239, the remaining thermal neutrons causing new fissions in uranium-238 thereby producing fast neutrons, a few of which produce additional fast neutrons by fission of uranium-238 atoms in the same body.
Some of the thermal neutrons entering uranium body A will cause fission in the uranium-235 to produce fast neutrons, the yield being at an average rate of about 2 neutrons per fission. As a result of this fission, fission fragments are released together with beta and gamma rays, producing energy which, in the system, is manifested mostly by the heating of the uranium bodies with only a slight release of heat to the graphite. The actual average yield of fast neutrons by fission of uranium-235 is slightly higher, by a few percent above the average of 2 mentioned above. Some of the fast neutrons released in the fission of uranium-235 by thermal neutrons almost immediately produce fast fission of uranium-238 in the same uranium body, with the production of additional fast neutrons.
The fast neutrons leaving the uranium body enter the mass of moderator and travel with paths that are long in comparison with the spacing of the uranium bodies. These neutrons will undergo successive collisions that slow them down. A substantial proportion of the fast neutrons are thus destined to be reduced, by about 100 elastic collisions apiece in the case of graphite and mostly in the moderator, to thermal energy. During this travel, before the neutrons arrive at thermal energies, a small percentage of the higher energy neutrons on the average may leak out of the system because of the finite size of the reactor, and be lost to the chain reaction. Furthermore, during the extremely irregular path of the neutrons while they are being slowed down by elastic collisions in the graphite, some of the neutrons will reach a uranium resonance absorption energy as they are about to enter a uranium body (such as C), and are absorbed immediately on or close to the surface of the uranium body. In addition some neutrons are reduced to resonance energy after entering the uranium body by an elastic collision with the uranium, and are immediately absorbed within the uranium body. Irrespective of whether the neutron resource adsorption in uranium-238 is on the surface, or in the volume of the uranium body, element-94 is produced by the resonance absorption according to

A small amount of plutonium-240 may also be found, formed by addition of a neutron to plutonium-239. Capture of thermal neutrons by uranium-238 as indicated in bodes A and C, also results in production of element-94 by the same process.
The predominant isotope produced, plutonium-239, is a long-lived radioactive product with a half-life of about 20,000 years.
A large percentage of the original fast neutrons escape resonance capture and fast neutron leakage, and are reduced to thermal energy within the system. Of these thermal neutrons, a small number on the average may leak by diffusion out of the system and be lost from the chain reaction, leaving the remainder of the thermal neutrons diffusing through the moderator in condition to produce fission if they promptly enter uranium-235 or element-94 without being captured by any other material.
The fission reaction is:-
uranium-235 + neutron = A + B + n neutrons (on average)
A = 'light' fission fragment, e.g. Br, Kr, Rb, Sr, Y, Zr, Cb, Mo, Ru, Rh. Atomic Mass 83-99 inclusive, or Atomic Number 35-45 inclusive.
B = 'heavy' fission fragment, e.g. Sb, Te, I, Xe, Cs, Ba, La, Ce, Pr, Nd. Atomic Mass 127-141 inclusive, or Atomic Number 51-60 inclusive.
In any practical system, impurities will be present in both the moderator and the uranium. In the chain described, a small fraction of neutrons can be captured and absorbed by impurities in the system without the reproduction factor of the system falling below unity. Also many of the thermal neutrons diffusing through the moderator are not in a position to promptly enter a uranium mass when they reach thermal energy, these thermal neutrons must continue to diffuse through the moderator until they do reach a uranium body. During this diffusion, a small percentage of the neutrons are absorbed by the moderator, leaving sufficient thermal neutrons to enter a uranium body to produce new fast neutrons by fission, to repeat the cycle. In a uranium-graphite system for every 100 fast neutrons about 72 thermal neutrons enter the uranium body to produce 100 new fast neutrons, i.e. a survival of about 72% of the original 100 fast neutrons during the slowing process.
The four neutron losses from the chain reaction referred to in the above figure, where the resonance absorption at C and the friction of thermal neutrons absorbed by uranium-238 at I represent the uranium absorption losses. Losses due to impurities are represented at F, those due to absorption in the moderator at G, and the leakage losses due to the finite size of the system at B and E.
It is possible to substantially reduce uranium resonance absorption by using light elements for moderators, few collisions are required to slow the neutrons to thermal energies with larger increments of energy loss per collision, thus decreasing the probability of a neutron being at a resonance energy as it enters a uranium atom. During the moderation, however, neutrons are moving through the slowing medium over random paths and distances so that the uranium is not only exposed to thermal neutrons but also to neutrons of energies varying between the energy of fission and thermal energy. Neutrons at uranium resonance energies will, if they enter uranium at these energies, be absorbed on the surface of a uranium body whatever its size, giving rise to surface absorption. And substantial reduction of overall surface of the same amount of uranium will reduce surface absorption, and any such reduction in surface absorption will release neutrons to enter directly into the chain reaction.
Neutrons are also subject to capture by the moderator. While carbon and beryllium have very small capture cross sections for thermal neutrons, and deuterium still smaller, a fraction of the thermal neutrons present in the system even under best conditions is lost by capture in the moderator during diffusion. It is desirable to have the neutrons reaching thermal energy enter uranium as promptly as possible. This may be taken care of by using optimum or near optimum geometry where the resonance absorption is substantially equal to absorption in the moderator.
However, even when resonance and moderator losses are educed to a practical minimum, no self-sustaining chain reaction can be obtained in any system unless impurities in the materials used for the reaction are reduced to such an extent that the loss by parasitic capture, together with other losses, prevent the reaction from becoming self-sustaining. Impurities present in both the uranium and the moderator consequently constitute a very important neutron loss factor in the chain. The effectiveness of various elements as neutron absorbers varies tremendously.
In any chain reacting system, it is only when the system has infinite size the there will be no exterior leakage of neutrons from the system. For any reactor of finite size exterior neutron losses will occur, and these losses will increase as the size of the reactor decreases.
Production Facilities
Right from the onset of the Manhattan Project the focus was always on doing what was need to obtain a weapon ready for the present war. It was a race against time. When in October 1942 the theoreticians suggested that the need for fissionable material might have doubled, time became even more important. Building small-scale plants would take too long. The Army wanted an industrial complex that could produce a number of weapons. Was there a short-cut? Could they go from laboratory to full-scale without the pilot plants? At the time the 'weakest' option was probably the building of reactors and in particular the large-scale separation of plutonium for irradiate uranium.
On October 3, 1942 a contract was signed with Du Pont to design and procure equipment for the separation plant. On November 10, 1942 Groves and Connant decided to bypass an electro-magnetic pilot plant and go for a full-scale plant.
Groves had already contracted Kellogg to plan for a full-scale gaseous-diffusion pilot plant (and that meant eliminating the centrifuge option). As the challenged started to weigh on Kellogg they decided to create the Kellex Company as a separate entity with its own accounts and name, ready to bear the stigmal of possible failure. Already in January 18, 1943 Kellogg 'forced' Groves to also decide on a site operator, and he picked Union Carbide and Carbon Corporation (we will just call them Union Carbide).
On November 12, 1942 it was also decided that Du Pont, would not only develop the electro-magnetic plant but also the full-scale pile. They took on this project without any assurances that it would be successful. The self-sustaining chain reaction had not been demonstrated, they did not know about the thermal stability of the reaction, there was no workable designs, and the recovery of plutonium from highly radioactive material had not been demonstrated on more than the micro-gram scale. On top of that everything was to work within 2 years. Looking back Du Pont took on the task because they became convinced that the pile and plutonium recovery was the best bet.
The below presentations of the industrial sites at Oak Ridge, Hanford, and Los Alamos are in an abbreviated form. I intend to dedicate a separate webpage to each site (sometime in the future).
Oak Ridge
In parallel with the building and testing of the pile in Chicago, work was on-going on the east Tennessee site where the first production facilities were to be built. Late 1942 saw the acquisition of a ninety-square-mile parcel (59,000 acres) in the ridges just west of Knoxville. Then started extensive site preparation to provide the transportation, communications, and utility needs of the town and production plants that would occupy the previously underdeveloped area. Original plans called for the Clinton Engineer Works, as the military reservation was named, to house approximately 13,000 people in 3,000 prefabricated housing, 1,000 trailers, and several wooden dormitories.
The name Oak Ridge did not come into usage until after WW II, but will be used here to avoid confusion.

Aerial view of the town of Oak Ridge looking east. In September 1942, General Leslie Groves, U.S. Army Corps of Engineers, designated a 59,000-acre parcel of land between Black Oak Ridge to the north and the Clinch River to the south as the first federal reserve for producing nuclear materials for the atomic bomb. Framed by the foothills of the Appalachian Mountains, the site originally consisted of 3,000 residents on family farms and in small communities.
The Manhattan Engineer District headquarters were moved from Washington to Tennessee in the summer of 1943 (Groves kept the Manhattan Project’s office in Washington).
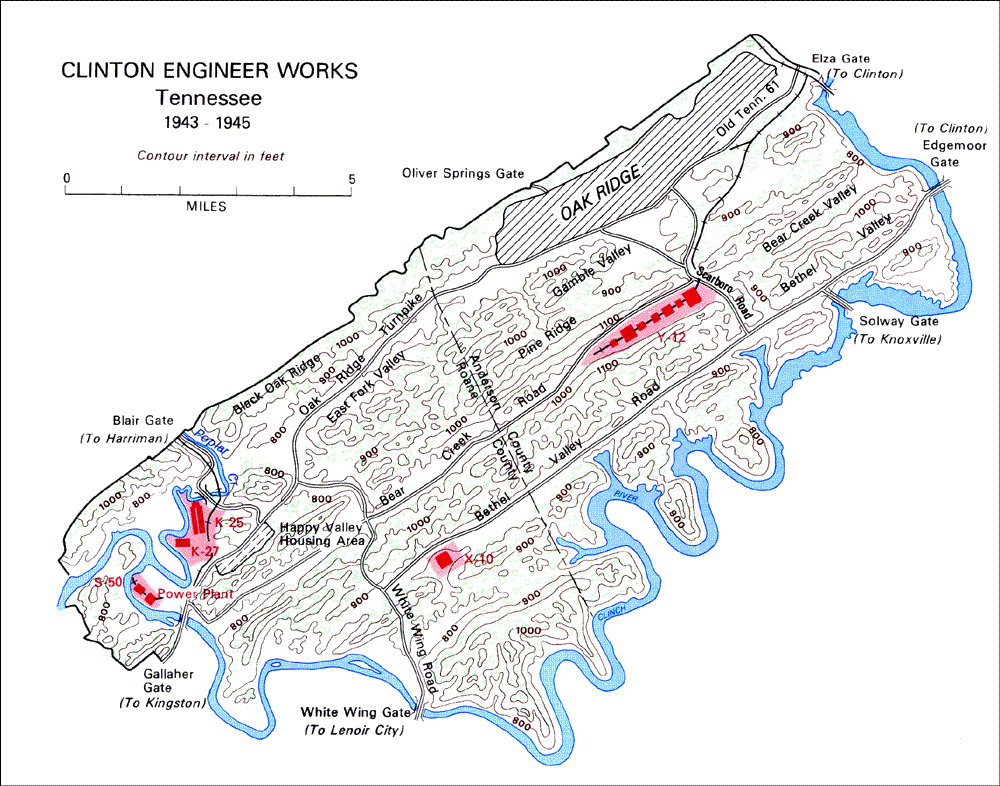
It was estimated that the town of Oak Ridge would be home to 40-45,000 people, but by May 1945, it was home to 82,000 people. Within months rural roads were converted into four-lane highways carrying more than 10,000 cars per day. Almost 100 miles of paved streets and more than 200 miles of pavements were laid so people could get to the production plants isolated in outlying valleys on the site. By end-1943 the budget allocated for the town was $24 million and $492 million was spent on the production areas.
At the end of the war, Oak Ridge was the fifth largest town in Tennessee, and the Clinton Engineer Works was consuming one-seventh of all the power being produced in the U.S. The three production facility sites were located in valleys away from the town. This provided security and containment in case of explosions. The Y-12 area, home of the electro-magnetic plant, was closest to Oak Ridge, being but one ridge away to the south. Farther to the south and west lay both the X-10 area, which contained the experimental plutonium pile and separation facilities, and K-25, site of the gaseous-diffusion plant and later the S-50 thermal diffusion plant. Y-12 and X-10 were begun early in 1943, and K-25 slightly later, but all three were well along by the end of the 1943. Below we can see Y-12 (immediately below) and X-10 (further below) in 1944.

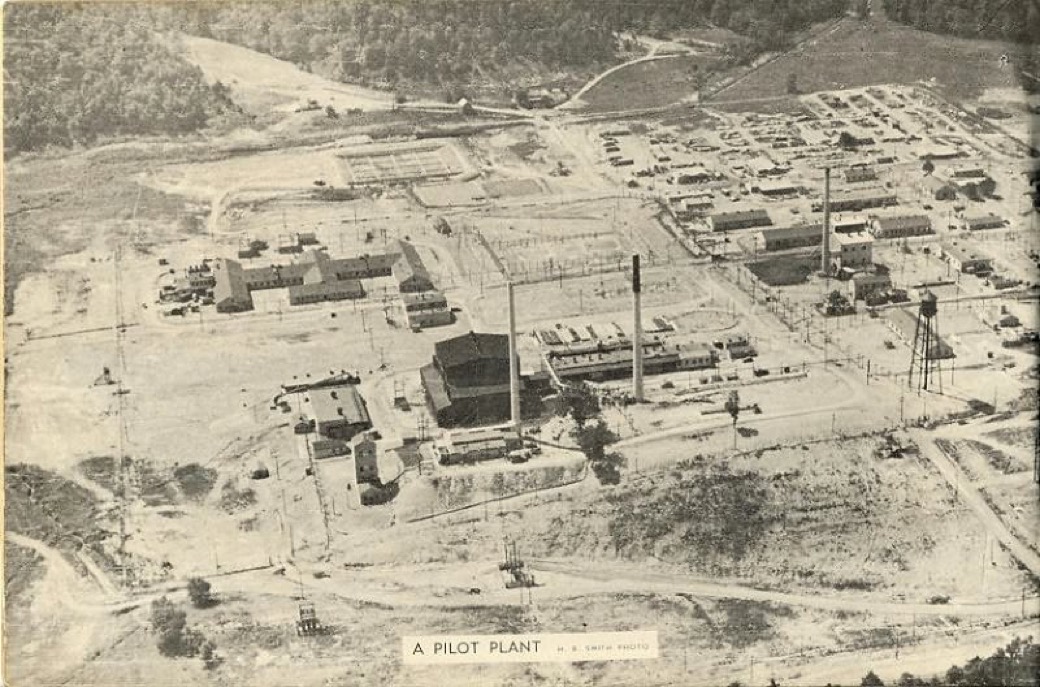
Although the Lewis report had placed gaseous-diffusion ahead of the electro-magnetic approach, many were still betting in early 1943 that Lawrence and his mass spectrograph would eventually predominate. Lawrence and his laboratory team at Berkeley continued to experiment with the giant 184-inch magnet, trying to reach a consensus on which shims, sources, and collectors to incorporate into Y-12 design for the Oak Ridge plant. Research on magnet size and placement and beam resolution eventually led to a “racetrack” configuration of two magnets with forty-eight gaps containing two vacuum tanks each. Ten such buildings were necessary to provide the 2,000 sources and collectors needed to separate 100 grams of uranium-235 daily. It was hoped that improvements in calutron design, or placing multiple sources and collectors in each tank, might increase efficiency and reduce the number of tanks and buildings required, but experimental results were inconclusive even as Stone & Webster of Boston, the Y-12 contractor at Oak Ridge, prepared to break ground.
Tennessee Eastman Corporation (a subsidiary of Eastman Kodak) was the plant operator of Y-12 and the electro-magnetic equipment was manufactured by the Westinghouse Electric and Manufacturing Company, the Allis-Chalmers Manufacturing Company, and the Chapman Valve Manufacturing Company. General Electric agreed to provide electrical equipment.
On January 14, 1943 the requirement was for the first racetrack of 96 tanks to be finished by July 1, 1943, and all 500 tanks finished by end 1943. Each racetrack was 122 feet long (37 m), 77 feet wide (23 m), and 15 feet high (4.6 m), the actual tank design was still in flux, and there were no plans yet for the chemical extraction facilities.
On March 17, 1943, it was decided to separate the process into two stages. The purpose of the second stage was to take the enriched uranium-235 derived from several runs of the first stage and use it as the sole feed material for a second stage of racetracks containing tanks approximately haIf the size of those in the first. Groves approved this arrangement and work began on both the Alpha (first-stage) and Beta (second-stage) tracks.
Building the Alpha plant was started on February 18, 1943, and the Beta plants was started before the decision was made to adopt a 2-stage process. Huge amounts of material had to be obtained (38 million board feet of lumber, for instance), and the magnets needed so much copper for windings that the Army had to borrow almost 15,000 tons of silver bullion from the U.S. Treasury to fabricate into strips and wind on to coils as a substitute for copper. Treasury silver was also used to manufacture the busbars that ran around the top of the racetracks. Below we have the Alpha-1 racetrack and below that the Beta-3 racetrack.
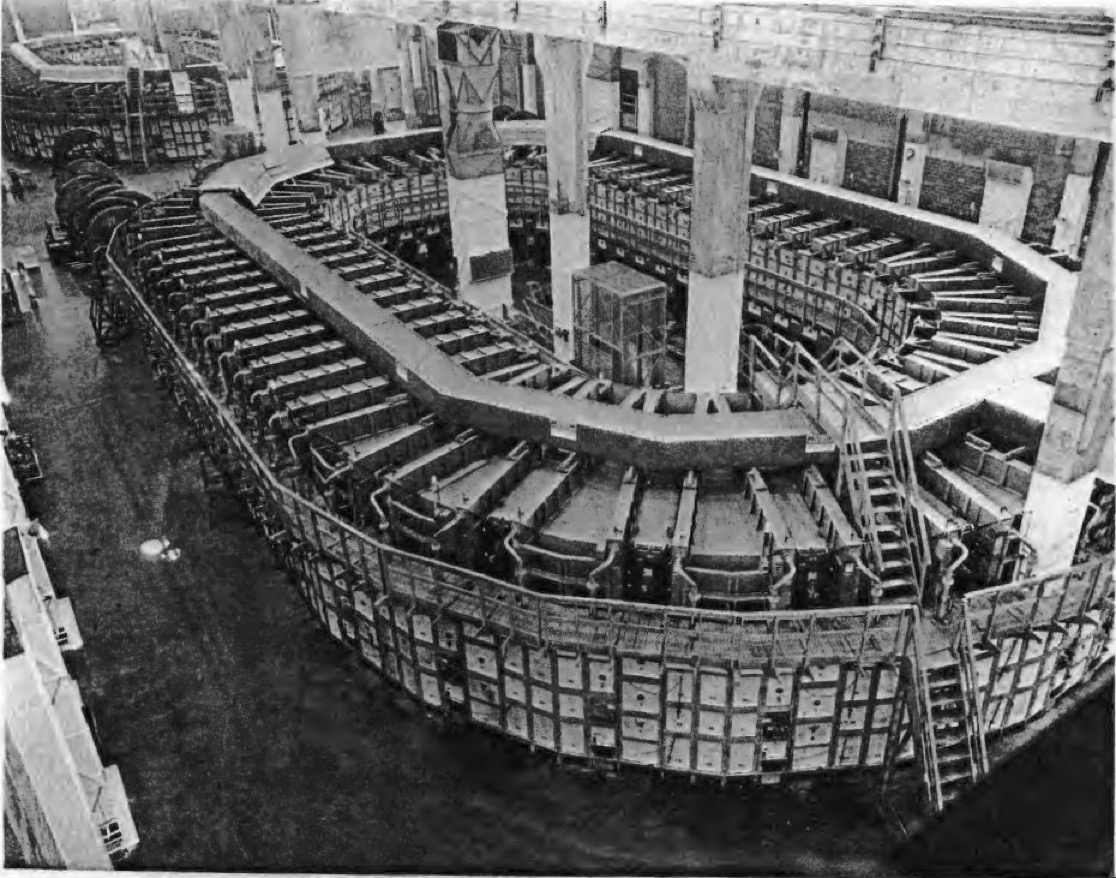
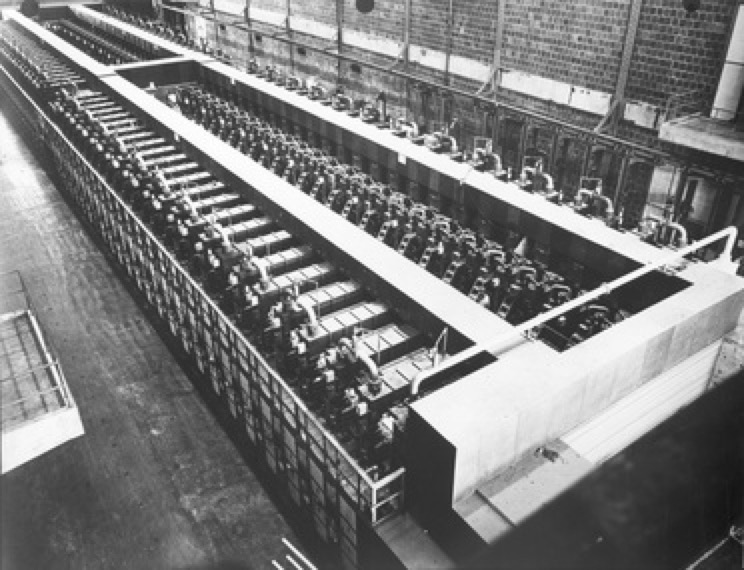
Improvements and changes were made on the fly. Lawrence found that hot (high positive voltage) electrical sources could replace the single cold (grounded) source, providing more efficient use of power, reducing insulator failure, and making it possible to use multiple rather than single beams. Meanwhile, receiver design evolved quickly enough in spring and summer 1943 to be incorporated into the Alpha plant. For a variety of reasons, including simplicity of maintenance, Tennessee Eastman decided that the Beta plant would consist of a rectangular, rather than oval, arrangement of two tracks of thirty-six tanks each.
About the same time Oppenheimer, in his new isolated laboratory in Los Alamos, warned that one might need three times as much fissionable material as had been originally indicated. So the designed Alpha and Beta racetracks might not do the job. Lawrence added additional beams to the design (4-beam instead of 1-beam), and convinced Groves to double the size of the complex with two new buildings, each with two rectangular racetracks with 96 tanks operating with 4-beam sources.
Along with the building at Oak Ridge, 5,000 people were also recruited to operate and maintain the new facilities. Vacuum tanks in the first Alpha racetrack leaked and shimmied out of line due to changes in the magnetic fields, welds failing, electrical circuits malfunctioning, and operators making frequent mistakes. Most seriously, the magnet coils shorted out because of rust and sediment in the cooling oil.

The operators in Y-12 produced some of the most iconic photographs of the period, e.g. the calutron operators and the shift changes at Y-12.

By February 1944 Y-12 had only produced 200 grams of 12% enriched uranium. And K-25, the gaseous-diffusion plant, still had problems with the barriers. K-25 would not be able to produce sufficiently enriched material for Y-12, without it being redesigned and retooled. An additional 4 racetracks would also be need, in addition to the 9 already finished or under construction.
If was finally decided to throw everything behind a new 30-beam calutron design.
From 1943 General Groves continued to carry the major responsibility for correlating the whole effort and keeping it directed toward its military objectives. His duty was to keep the various parts of the project in step, to see that raw materials were available for the various plants, to determine production schedules, to make sure that the development of bomb design kept up with production schedules, to arrange for use of the bombs when the time came, and to maintain an adequate system of security. Brigadier General Thomas Francis Farrell acted as General Groves' deputy in the important later phases of the project. Colonel Kenneth David Nichols was the District Engineer of the Manhattan District with his headquarters at the Clinton Engineer Works. He was concerned with the research and production problems of both uranium-235 and plutonium. Colonel Franklin Thompson Matthias was responsible for both the construction and operational phases at the Hanford Engineer Works. Colonel Stafford Leak Warren was the chief of the Medical Section of the Manhattan District.
The approach taken by Lawrence and his team in developing the cyclotron through to the calutron was almost purely experimental. Some parts were based upon theory, but a lot of the work with emitters, shims, and collectors was purely experimental. Tests were made, equipment had windows in them so photographs could be taken, changes were made, and tests were repeated. In the move from the laboratory to Y-12 the biggest effort in what might be considered 'classical' experimentation was with the magnets. Below we can see the 184-inch 'laboratory' magnet and the Beta racetrack magnets in Y-12.
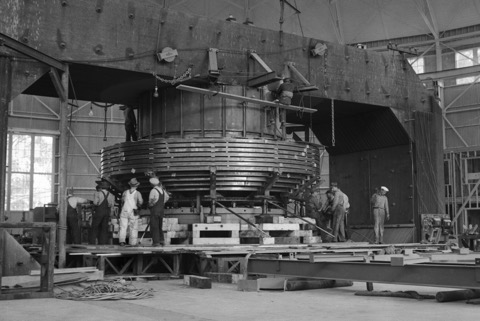
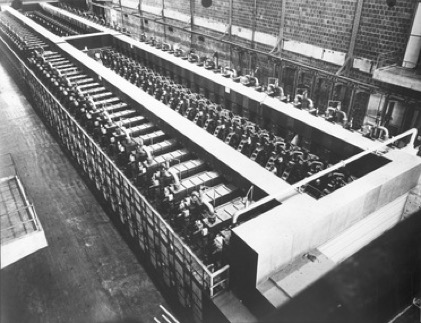
The theory of magnets was well established, and the immediate assumption was that an industrial plant would use a large magnet with a large number of gaps. Theory showed that as the number of gaps increased, the weight of the steel in the magnet yokes increased, as did the weight of the coils, but the power requirement per gap decreased to a constant value. In theory the bigger the better, but the limiting factors became the amount of power that could be delivered to a magnet, the size of the building, and above all the risk of production loss if a magnet failed. Smaller magnets needed more material, more power, but a failure would not close down the whole plant. So a compromise was needed.
As you can imagine vertically stacking gaps in a magnet looked to be problem, so the decision was to line up vertical gaps in a horizontal magnetic field. The tanks would stand on end and the ion beams would be vertical, perpendicular to a horizontal magnetic field. To obtain a practical separation of the two beams (uranium-235 and uranium-238) a beam radius of about 120 cm would be needed, so the cross section of the magnet core would need to be about 250 cm. In a properly shimmed field, the width of the magnetic gap could be about ½ a beam radius (60 cm), and therefore a magnetic field of about 0.35 Tesla would be needed. This magnetic field strength is about the same as found in a modern-day magnetic resonance medical diagnostic machine (MRI).
Given the performance of the 184-inch calutron in 1942 the initial assessment was that 2,000 tanks would be needed to enrich 100 gm/day. The contractor came up with a machine (a 'racetrack') with two magnets and two tanks in each of 48 gaps.
The tanks needed to have a very good vacuum, and all water vapour removed. In the laboratory a vacuum was a relatively commonplace feature, but a 'racetrack' required an industrial-scale vacuum which was extremely rare at the time. The first problem was reliability of the pumps over long-term operation, the second problem was just the size of the vacuum, the third problem was that gaskets and welds had to withstand large temperature variations and long periods of operation, and the final problem was that the tanks would be opened after each run, so the pumps would need to restore quickly the vacuum again.
Building 10 separate racetracks looked to be a daunting exercise, so Lawrence tried to find short-cuts, and one of them was to use two sets of double sources and collectors per tank. This required a redesigning of the shims, and was coupled with a new design incorporating the source, liner, and receiver as one unit that could be removed and replaced as a single unit.
At the time the approach was essentially experimental, without a true theoretical foundation for the process. For example tests with a three-electrode source produced an improved beam but no one understood why. Trial and error brought the number of tanks down to 1,000 and the number of building down from ten to five. And again it was trial and error that found the optimal positioning of the slits, etc. And not one of the laboratory calutrons looked anything like the industrial version being designed by Stone & Webster.
The story continues more or less word-for-word from “The Manhattan Project: Making the Atomic Bomb”, by F.G. Gosling (1999), report DOE/MA-0001-01/99.
Eleven miles southwest of Oak Ridge on the Clinch River was the site of the K-25 gaseous-diffusion plant. Championed by the British and placed first by the Lewis committee, gaseous-diffusion seemed to be based on sound theory but had not yet produced samples of enriched uranium-235. At Oak Ridge, on a relatively flat area of about 5,000 acres, site preparation for the K-25 power plant began in June, 1943. Throughout the summer, contractors contended with primitive roads as they shipped in the materials needed to build what became the world’s largest steam electric plant. In September 1943 work began on the cascade building (below), plans for which had changed dramatically since that spring.
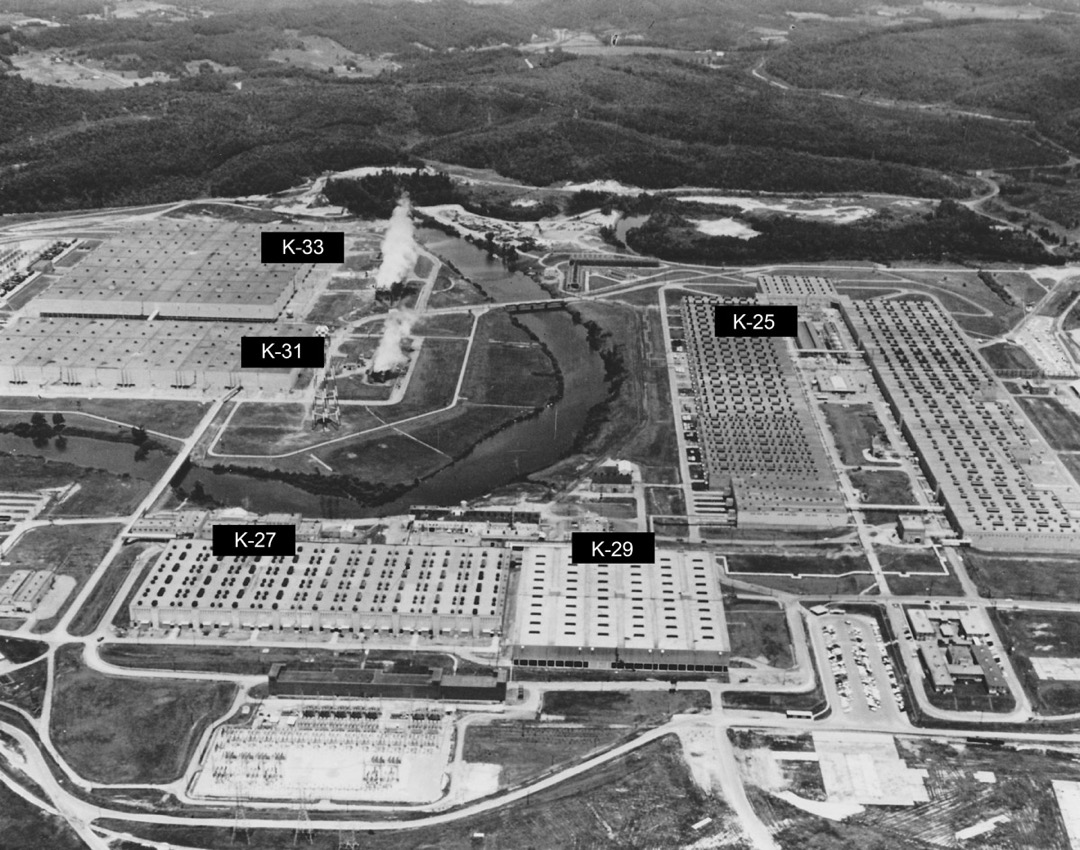
K-25 is the original gaseous-diffusion plant, and at the time of its construction it was the world’s largest building. K-27, K-29, K-31, and K-33 were additional gaseous-diffusion plants added after WW II. The plants ceased to operate in 1985, and was up for demolition in 2016.
Plans show that there were to be fifty four-story buildings (2,000,000 square feet) in a U-shape measuring half a mile by 1,000 feet. Innovative foundation techniques were required to avoid setting thousands of concrete piers to support load-bearing walls.
Since it was eleven miles from the headquarters at Oak Ridge, the K-25 site developed into a satellite town. Housing was supplied, as was a full array of service facilities for the population that reached 15,000. Dubbed sardonically Happy Valley by the inhabitants, the town had housing similar to that in Oak Ridge (so-called 'hutments' and barracks and trailers).

On December 10, 1942 it was agreed that K-25, the full-scale gaseous-diffusion plant, would be the first to be built. In late summer 1943 it was decided that K-25 would play a lesser role than originally intended. Instead of producing fully enriched uranium-235, the gaseous-diffusion plant would now provide around 50% enrichment for use as feed material in Y-12. This would be accomplished by eliminating the more troublesome upper part of the cascade. Even this level of enrichment was not assured since a barrier for the diffusion plant still dld not exist. The decision to downgrade K-25 was part of the larger decision to double Y-12’s capacity and it fit with Groves’s new strategy of utilising a combination of methods to produce enough fissionable material for bombs as soon as possible.
In summer 1943 Urey was increasingly troubled by frequent snags in the gaseous-diffusion process. They simply had not been able to produce a barrier to be used in K-25. This pessimistic attitude was in opposition to Lawrence who was 'selling' the electro-magnetic plant, despite the fact that Y-12 had not yet operated one production model of the calutron. Lawrence also stressed that the work on the electro-magnet plant Y-12 could be greatly advanced if partially enriched uranium from K-25 could be used as a feed material. This would eliminate the need for the top cascade on K-25. This was not a new idea, the 'squared-off' cascade had been studies in January 1943, and earlier it had been proposed to use the gaseous-diffusion output as input to centrifuges. In addition to the difficulties with the 'barrier' (membrane) they had also problems with the pumps and seals for the top of the cascade. By August 13, 1943 it was decided to limit the product enrichment of K-25 to something less than 50%, and use that as feed for Y-12. And at that time they had not even broken ground on the building of K-25, so it is not surprising that Groves also doubled the size of Y-12.
There was no doubt in Groves’s mind that gaseous-diffusion still had to be pursued vigorously. Not only had major resources already been expended on the program, but there was also the possibility that it might yet prove successful. Y-12 was in trouble as 1944 began, and the plutonium pile projects were just getting underway. A workable barrier design might put K-25 ahead in the race for the bomb. Unfortunately, no one had been able to fabricate a barrier of sufficient quality. The only alternative remaining was to increase production enough to compensate for the poor performance of the barrier.
Above we have a very rapid summary of the situation, too rapid and too superficial. I don't intend here to detail all the different problems encountered in the construction of K-25 but some details and precisions are worth noting. One thing is to outline an ideal cascade with a reasonable number of stages, etc., but it another to design a $100 million plant. The plant designers could imagine what the plant should be, but the basic components had not yet been designed. It was not clear how the uranium hexafluoride would travel through pumps, converters and hundreds of miles of pipes. Different types of barriers were tested, but pumps were almost as important (they wear and leak). Barriers were expected to be separative and able to work over long periods. How could you have sub-microscopic holes that would not block? The barrier had to be strong enough to be used in a large-scale plant operating under severe conditions. Was the nickel Norris-Adler barrier the answer? Would they not be too brittle and fragile? Hundreds of nickel barriers were developed, treated and discarded. Porosity, tensile strength, bending properties, resistance to fracture, corrosion resistance, occurrence of holes, resistance to plugging, all were factors.
In January 1943 they had already plans for a pilot plant to produce the barrier, but they did not know what to produce. A full-scale production plant for the barrier was planned, they would need several million square feet. Without a viable barrier the pilot plant was on hold, but the Clinton Engineering Works was selected as the site. On May 31, 1943 the engineers started to project the huge power plant that would be needed. It would be the worlds largest steam-electric power plant. The building would be enormous, and with the machinery, would require specially excavated foundations (they moved 3 million cubic metres of earth).
With time barriers improved, but the manufacturing process was unreliable. A new alternative in the form of a nickel power barrier emerged. A new process, the Bakelite process, was developed. Clarence A. Johnson working for Kellex developed a new barrier, and it was decided to develop both options.
Barriers were one problem but orders had already been sent out for half a million valves of different sizes, tens on thousands of recording instruments, gauges, mass spectrometers, pressure indicators, flowmeters, thermometers, and compressors. Process piping values, pumps and converters were being installed in the K-25 building. The building had employed nearly 20,000 people and had cost $281 million, but they were waiting for the barriers.
The Problems Mounted Up
So there were problems with both Y-12 and K-25. President Roosevelt had instructed that the atomic bomb effort be an Army program and that the Navy be excluded from deliberations. Navy research on atomic power, conducted primarily for submarines, received no direct aid from Groves, who, in fact, was not up-to-date on the state of Navy efforts when he received a letter on the subject from Oppenheimer late in April 1944.
Oppenheimer informed Groves that Philip Abelson’s experiments on thermal diffusion at the Philadelphia Naval Yard deserved a closer look. The liquid thermal diffusion process had been evaluated in 1940 by the Uranium Committee, when Abelson was at the National Bureau of Standards. In 1941 he moved to the Naval Research Laboratory, where there was more support for his work. During summer 1942 Bush and Conant received reports about Abelson’s research but concluded that it would take too long for the thermal diffusion process to make a major contribution to the bomb effort, especially since the electro-magnetic and pile projects were making satisfactory progress. So Abelson continued his work independently of the Manhattan Project. Groves immediately saw the value of Oppenheimer’s remarks, and a quick analysis demonstrated that a thermal diffusion plant could be built at Oak Ridge and placed in operation by early 1945. The steam needed in the convection columns was already at hand in the form of the almost completed K-25 power plant. It would be a relatively simple matter to provide steam to the thermal diffusion plant and produce enriched uranium, while providing electricity for the K-25 plant when it was finished. Groves gave the contractor, H. K. Ferguson Company of Cleveland, just ninety days from September 27, 1943 to bring S-50 (below), a 2,142-column plant on line (Abelson’s plant contained 100 columns).

S-50, the black building next to the power station, became the first stage of enrichment, achieving enrichment levels of under 2%. This material was fed into K-25 which achieved about 23% enrichment, and this was fed to the calutrons in Y-12, which achieved about 84% enrichment. The liquid thermal diffusion process required water (from the Clinch River) and steam from the new K-25 power plant. The cooling water for the process was drawn from the river using four pumps, each pulling 57,000 litres per minute. The K-25 power plant was initially available for S-50, but overtime K-25 demanded more and more power, so a new boiler plant was built for S-50. In fact 12 surplus boilers from navy ships were commandeered. Because these boilers were oil-fired a 23 million litre oil tank farm was built. Below we can see a few of the 16 meter tall columns.
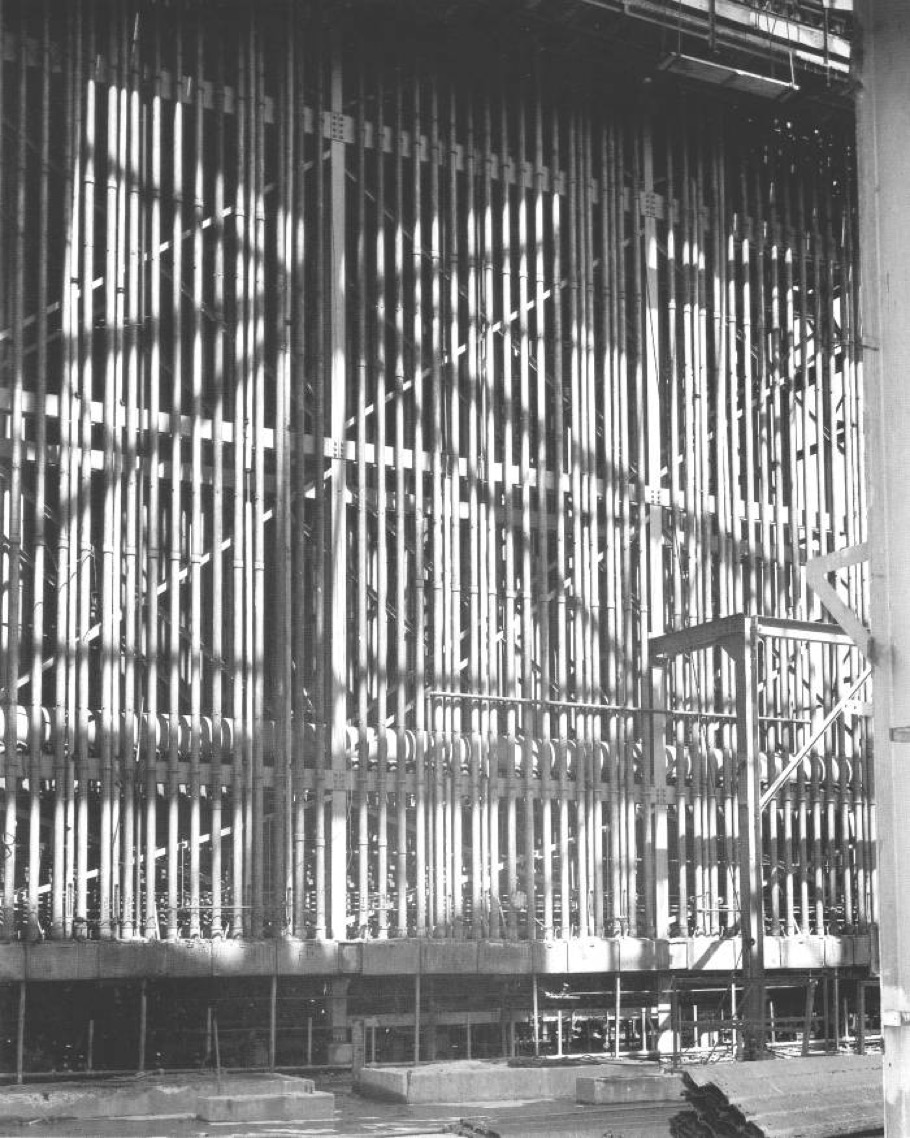
Below we have a part of the liquid thermal diffusion column taken from the U.S. patent for the process. Tube (10) is set inside another tube (11), and both maybe made of any heat transmitting solid. The inner tube has groves in pairs (16 and 16a), but they could be a single or a set of three. The groves are for O-rings (17) to seal the annular space (15). Steam or hot water is injected into the interior (14) through an entry tube (12). In the jacket (11a) another heat transfer medium is injected (20a), e.g. cold water. The product to be enriched enters and leaves through a plurality of pipes (example 21, 22, and 27, 28) effectively dividing the chamber (15) into several separation chambers.
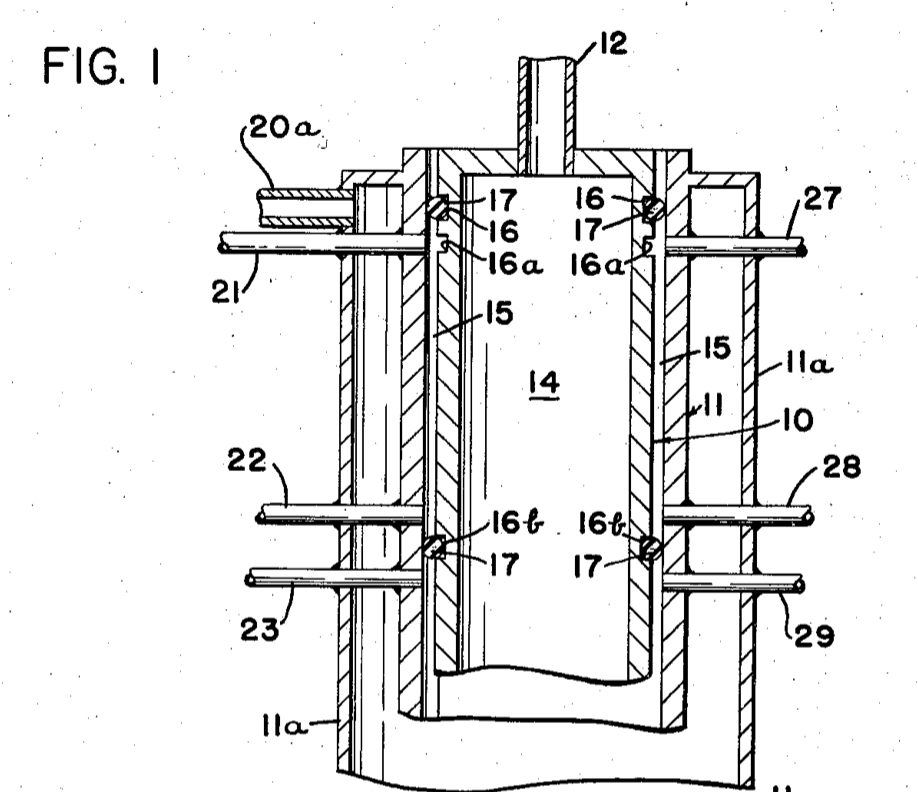
The production pile for plutonium
The Metallurgical Laboratory (Met Lab) in Chicago was counted on to design a production pile for plutonium. Here again the job was to design equipment for a technology that was not well understood even in the laboratory. The Fermi pile, important as it was historically, provided little technical guidance other than to suggest a lattice arrangement of graphite and uranium. Any pile producing more power than the few watts generated in Fermi’s famous experiment would require elaborate controls, radiation shielding, and a cooling system. These engineering features would all contribute to a reduction in neutron multiplication (neutron multiplication being represented by ƙ); so it was imperative to determine which pile design would be safe and controllable and still have a ƙ high enough to sustain an ongoing reaction.
A group headed by Compton’s chief engineer, Thomas V. Moore, began designing the production pile in June 1942. Moore’s first goals were to find the best methods of extracting plutonium from the irradiated uranium and for cooling the uranium. It quickly became clear that a production pile would differ significantly in design from Fermi’s experimental reactor, possibly extending uranium rods into and through the graphite next to cooling tubes and building a radiation and containment shield. Although experimental reactors like Fermi’s didn't generate enough power to need cooling systems, piles built to produce plutonium would operate at higher power levels and require coolants. The Met Lab group considered the full range of gases and liquids in a search to isolate the substances with the best nuclear characteristics, with hydrogen and helium standing out among the gases and water (even with its marginal nuclear properties and tendency to corrode uranium) as the best liquid.
During the summer, Moore and his group began planning a helium-cooled pilot pile for the Argonne Forest Preserve near Chicago, built by Stone & Webster, and on September 25, 1942, they reported to Compton. The proposal was for a 460-ton cube of graphite to be pierced by 376 vertical columns containing twenty-two cartridges of uranium and graphite. Cooling would be provided by circulating helium from top to bottom through the pile. A wall of graphite surrounding the reactor would provide radiation containment, while a series of spherical segments that gave the design the nickname 'Mae West' would make up the outer shell.
By the time Compton received Moore’s report, he had two other pile designs to consider. One was a water-cooled model developed by Eugene Wigner and Gale Young, a former colleague of Compton’s. Wigner and Young proposed a twelve-foot by twenty-five-foot cylinder of graphite with pipes of uranium extending from a water tank above, through the cylinder, and into a second water tank underneath. Coolant would circulate continuously through the system, and corrosion would be minimised by coating interior surfaces or lining the uranium pipes.
I'm not sure, but below we have the design of a patent registered by Wigner. In this patent ordinary water is circulated through the uranium tubes (30) by means of an inlet line (42) comprising a pump (44) activated by a motor (46). The water is moved from an inlet chamber (6) to an outlet chamber (12) and is maintained under super atmospheric pressure in order to prevent vaporisation within the reactor.
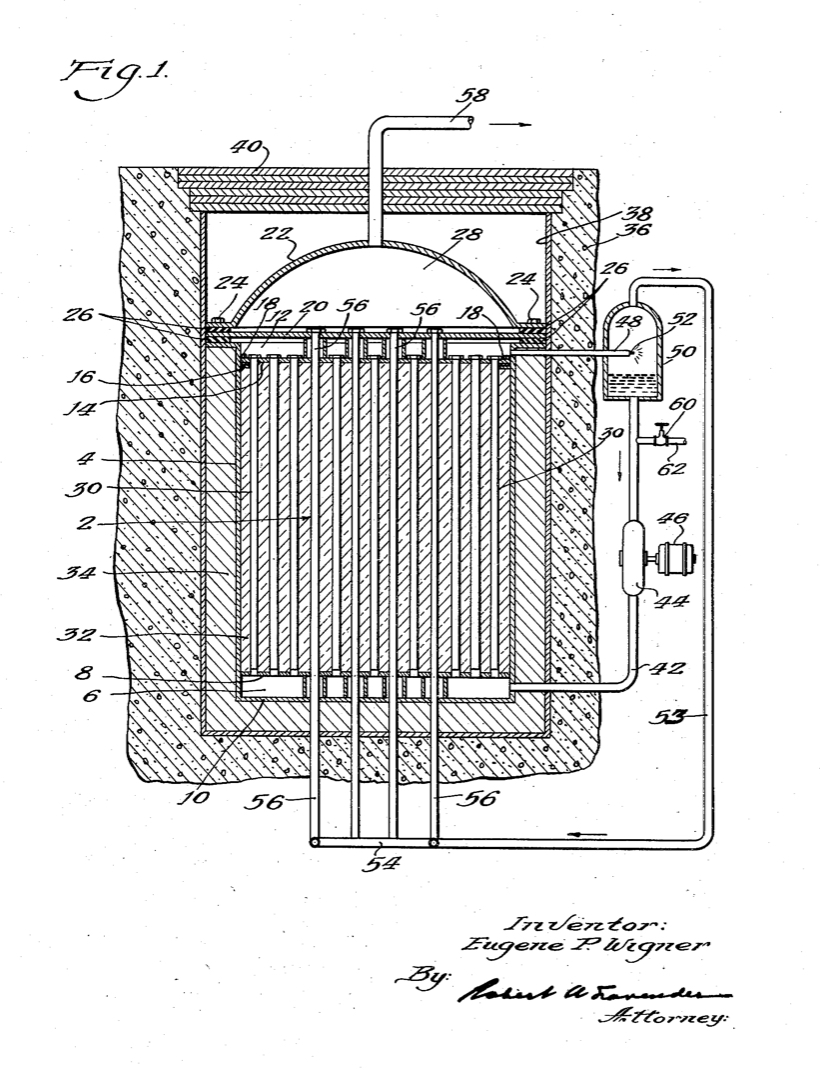
The other alternative to 'Mae West' was more daring. Szilárd thought that liquid metal would be such an efficient coolant that, in combination with an electro-magnetic pump having no moving parts (adapted from a design he and Einstein had created), it would be possible to achieve high power levels in a considerably smaller pile. Szilárd had trouble obtaining supplies for his experiment, primarily because bismuth, the metal he preferred as the coolant, was rare.
The reality is that both Einstein and Szilard knew about taking out patents. Einstein had been a patent officer in Switzerland, and had taken out a patent for a hearing air. Szilard patented a variety of things, from an x-ray sensitive cell to the nuclear reactor which he patented with Fermi. In the late 20's the new refrigerator when defaulty could release poisonous gases which could kill. They decided to work on an alternative, safe refrigerator design, and they invented the electro-magnetic pump. The idea was developed by A.E.G., but due to cavitation as the liquid metal passed through the pump, it was extremely noisy. It was patented and the idea was used, but not for a refrigerator. The basic idea has been used for cooling breeder reactors.
In October 1942 Groves was in Chicago ready to force a showdown on pile design. Szilárd was noisily complaining that decisions had to be made so that designs could move to procurement and construction. Compton’s delay reflected uncertainty on the superiority of the helium pile and awareness that engineering studies could not be definitive until the precise value of ƙ had been established. Some scientists at the Met Lab urged that a full production pile be built immediately, while others advocated a multi-step process, perhaps beginning with an externally cooled reactor proposed by Fermi.
On October 5, 1943 Groves insisted that the Met Lab decide on pile design within a week. Even wrong decisions were better than no decisions, Groves claimed, and since time was more valuable than money, more than one approach should be pursued if no single design stood out. Compton decided on compromise. Fermi would study the fundamentals of pile operation on a small experimental unit to be completed and in operation by the end of 1943. Hopefully he could determine the precise value of ƙ and make a significant advance in pile engineering possible. An intermediate pile with external cooling would be built at Argonne and operated until June 1, 1943, when it would be taken down for plutonium extraction. The helium cooled “Mae West”, designed to produce 100 grams of plutonium a day, would be built and operating by March 1944. Studies on liquid-cooled reactors would continue, including Szilárd’s work on liquid metals.
In practical terms, today there are two different kinds of plutonium: reactor-grade and weapons-grade. The first is recovered as a by-product of used fuel from a nuclear reactor, after the fuel has been irradiated ('burned') for about three years. The second is made specially for military purposes, and is recovered (separated or reprocessed) from uranium fuel that has been irradiated for only 2-3 months in a plutonium production reactor. The two types of plutonium differ in their isotopic composition, but both are proliferation risks.
The most common isotope formed in a typical nuclear reactor is the fissile plutonium-239 isotope, formed by neutron capture from uranium-238 (followed by beta decay), and which yields much the same energy as the fission of uranium-235. Well over half of the plutonium created in the reactor core is 'burned' in situ and is responsible for about one third of the total heat output of a typical “light water” power reactor (LWR). Of the rest, about one sixth through neutron capture becomes plutonium-240 (and plutonium-241). The approximately 1.15% of plutonium in the spent fuel removed from a commercial LWR power reactor (burn-up of 42 GWd/t) consists of about 53% plutonium-239, 25% plutonium-240, 15% plutonium-241, 5% plutonium-242 and 2% of plutonium-238, which is the main source of heat and radioactivity.
In a reactor producing weapon plutonium, the burn-up might be about 0.4 GWd/t (so about 3 months irradiation and not 3 years), and a typical plutonium isotope vector might be about 80% plutonium-239, 16.9% plutonium-240, 2.7% plutonium-241, 0.3% plutonium-242 and 0.1% of plutonium-238.
Today a “typical” 1000 MWe light water reactor produces about 25 tons of used fuel a year, containing up to 290 kilograms of plutonium. If the plutonium is extracted from used reactor fuel it can be used as a direct substitute for uranium-235 in the usual fuel, the plutonium-239 being the main fissile part, but plutonium-241 also contributing. In order to extract it for recycle, the used fuel is reprocessed and the recovered plutonium oxide is mixed with depleted uranium oxide to produce a so-called mixed-oxide (MOX) fuel, with about 8% Pu-239 (this corresponds with uranium enriched to 5% U-235). This plutonium can also be used fast breeder reactors, and is the preferred fuel for the future IV generation nuclear reactors.
As a best estimate, total world generation of reactor-grade plutonium in spent fuel is some 70 tons per year. About 1,300 tons have been produced so far, and most of this remains in the used fuel, with some 370 tons having been extracted. About one-third of the separated plutonium (130 tons) has been used in MOX fuel over the last 30 years. Over 20 tons of reactor-grade plutonium is separated by reprocessing plants in the OECD each year and this is set to increase. Eventually its usage in MOX is expected to outstrip this level of production so that stockpiles diminish.
At the end of 2013 the UK plutonium stockpile had 123 tons of separated civil plutonium from historic and current operations and foreign swaps. It includes some 83 tons from Magnox fuel, 15 tons from AGR fuel and 15 tons foreign-owned. On completion of reprocessing operations (about 2016) the stockpile is expected to be 140 tons. Using all of UK's plutonium in MOX fuel rather than immobilising it as waste is expected to yield a £700-1200 million resource cost saving to UK, along with over 700 billion kWh of electricity (about two years' UK supply).
At the end of 2010 France held 80 tons of separated civil plutonium, 60 tons of it at La Hague. Some 10.5 tons of plutonium and 1,000 tons of reprocessed uranium are recovered each year from the 1,050 tonnes treated each year. The plutonium is immediately shipped to the 195 tons/yr Melox plant near Marcoule for prompt fabrication into about 100 tonnes of mixed-oxide (MOX) fuel.
Russia holds at least 32 tons from reprocessing power reactor fuel (and 34 tons of weapons-grade plutonium from military stockpiles to be used in MOX fuel for BN-600 and BN-800 fast neutron reactors at Beloyarsk, supported by a $400 million payment from the USA).
The U.S. has no reactor-grade plutonium separated, but at least 34 tons of weapons-grade material is destined for MOX. China has no reactor-grade plutonium separated. India’s plutonium stocks are unknown. Worldwide stocks of civil plutonium are estimated as around 260 tons.
Continued disarmament is likely to give rise to some 150-200 tons of weapons-grade plutonium, over half of it in Russia. Most of this is likely to be used in MOX for existing or fast neutron reactors. In June 2000, the U.S. and Russia agreed to dispose of 34 tons each of weapons-grade plutonium by 2014, and since then the U.S. government has released further surplus weapons plutonium (the 2000 agreement was reaffirmed in 2010). Construction on the Mixed Oxide Fuel Fabrication Facility at the Savannah River Site near Aiken, South Carolina commenced in Aug. 2007 (it has a operational license since 2014). The plant is designed to convert 3.5 tons/yr of weapons-grade plutonium into mixed oxide (MOX) fuel. Initial trials of MOX fuel made with weapons plutonium have been successful. Russia plans to continue to use all its military plutonium in fast-neutron reactors.
The situation described above was valid for 2016
Transuranium chemistry
While the Met Lab laboured to make headway on pile design, Seaborg and his coworkers tried to gain enough information about transuranium chemistry to insure that plutonium produced could be successfully extracted from the irradiated uranium. Using lanthanum fluoride as a carrier, Seaborg isolated a weighable sample of plutonium in August 1942. At the same time, Isadore Perlman and William J. Knox explored the peroxide method of separation; John E. Willard studied various materials to determine which best adsorbed plutonium; Theodore T. Magel and Daniel K. Koshland, Jr., researched solvent-extraction processes; and Harrison Scott Brown and Orville F. Hill performed experiments into volatility reactions.
Basic research on plutonium’s chemistry continued as did work on radiation and fission products. Seaborg’s discovery and subsequent isolation of plutonium were major events in the history of chemistry, but, like Fermi’s achievement, it remained to be seen whether they could be translated into a production process useful to the bomb effort. In fact, Seaborg’s challenge seemed even more daunting, for while piles had to be scaled up ten to twenty times, a separation plant for plutonium would involve a scale-up of the laboratory experiment on the order of a billion-fold.
PUREX is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. It is an acronym standing for Plutonium Uranium Redox EXtraction. PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used ("spent", or "depleted") nuclear fuel. It is based on liquid–liquid extraction ion-exchange.
The PUREX process was invented by Herbert H. Anderson and Larned Brown Asprey at the Metallurgical Laboratory at the University of Chicago, as part of the Manhattan Project under Seaborg; their patent "Solvent Extraction Process for Plutonium" filed in 1947, mentions tributyl phosphate as the major reactant which accomplishes the bulk of the chemical extraction.
What we see above is a later patient for a method that successively comprises: a) co-extracting the uranium (VI), plutonium (IV) and other actinides (IV) or (VI) from an aqueous nitric solution by using solvent phase and scrubbing the latter; b) back-extracting the plutonium in oxidation state (III) from the solvent phase by using an aqueous nitric solution; c) back-extracting the uranium in oxidation state (VI) from the solvent phase by using an aqueous nitric solution; d) concentrating the aqueous nitric solution resulting from step c) with respect to uranium (VI); and it is characterised in that some of the uranium (VI)-concentrated aqueous solution obtained in step d) is used for back-extracting the actinide (IV) or actinides (IV) from the solvent phase during step b) or between steps b) and c).
Collaboration with Du Pont’s Charles Milton Cooper and his staff on plutonium separation facilities began even before Seaborg succeeded in isolating a sample of plutonium. Seaborg was reluctant to drop any of the approaches then under consideration, and Cooper agreed. The two decided to pursue all four methods of plutonium separation but put first priority on the lanthanum fluoride process Seaborg had already developed. Cooper’s staff ran into problems with the lanthanum fluoride method in late 1942, but by then Seaborg had become interested in phosphate carriers. Work led by Stanley Gerald Thompson found that bismuth phosphate retained over ninety-eight percent plutonium in a precipitate. With bismuth phosphate as a backup for the lanthanum fluoride, Cooper moved ahead on a semi-works near Stagg Field.
Compton’s original plans to build the experimental pile and chemical separation plant on the University of Chicago campus changed during fall 1942. It was decided that it would be safer to put Fermi’s pile in Argonne Forest and build the pilot plant and separation facilities in Oak Ridge than to place these experiments in a populous area. On October 3, 1942, Du Pont agreed to design and build the chemical separation plant. Groves tried to entice further Du Pont participation at Oak Ridge by having the firm prepare an appraisal of the pile project and by placing three Du Pont staff members on the Lewis committee. Because Du Pont was sensitive about its public image (the company was still smarting from charges that it profiteered during WW I), Groves ultimately obtained the services of the giant chemical company for the sum of one dollar over actual costs. In addition, Du Pont vowed to stay out of the bomb business after the war and offered all patents to the United States government.
Groves had done well in convincing Du Pont to join the Manhattan Project. Du Pont’s proven administrative structure assured excellent coordination (Crawford Greenewalt was given the responsibility of coordinating Du Pont and Met Lab planning), and Groves and Compton welcomed the company’s demand that it be put in full charge of the Oak Ridge plutonium project. Du Pont had a strong organisation and had studied every aspect of the Met Lab’s program thoroughly before accepting the assignment. While deeply involved in the overall war effort, Du Pont expected to be able to divert personnel and other resources from explosives work in time to throw its full weight into the Oak Ridge project.
For those interested, Seaborg registered a U.S. patent for a 'method for producing, separating, and purifying plutonium'.
Hanford Engineering Works
Moving the pilot plutonium plant to Oak Ridge left too little room for the full-scale production plant at the X-10 site and also left too little generating power for yet another major facility. Furthermore, the site was uncomfortably close to Knoxville should a catastrophe occur. Thus the search for an alternate location for the full-scale plutonium facility began soon after Du Pont joined the production team. Compton’s scientists needed an area of approximately 225 square miles. Three or four piles and one or two chemical separation complexes would be at least a mile apart for security purposes, while nothing would be allowed within four miles of the separation complexes for fear of radioactive accidents.
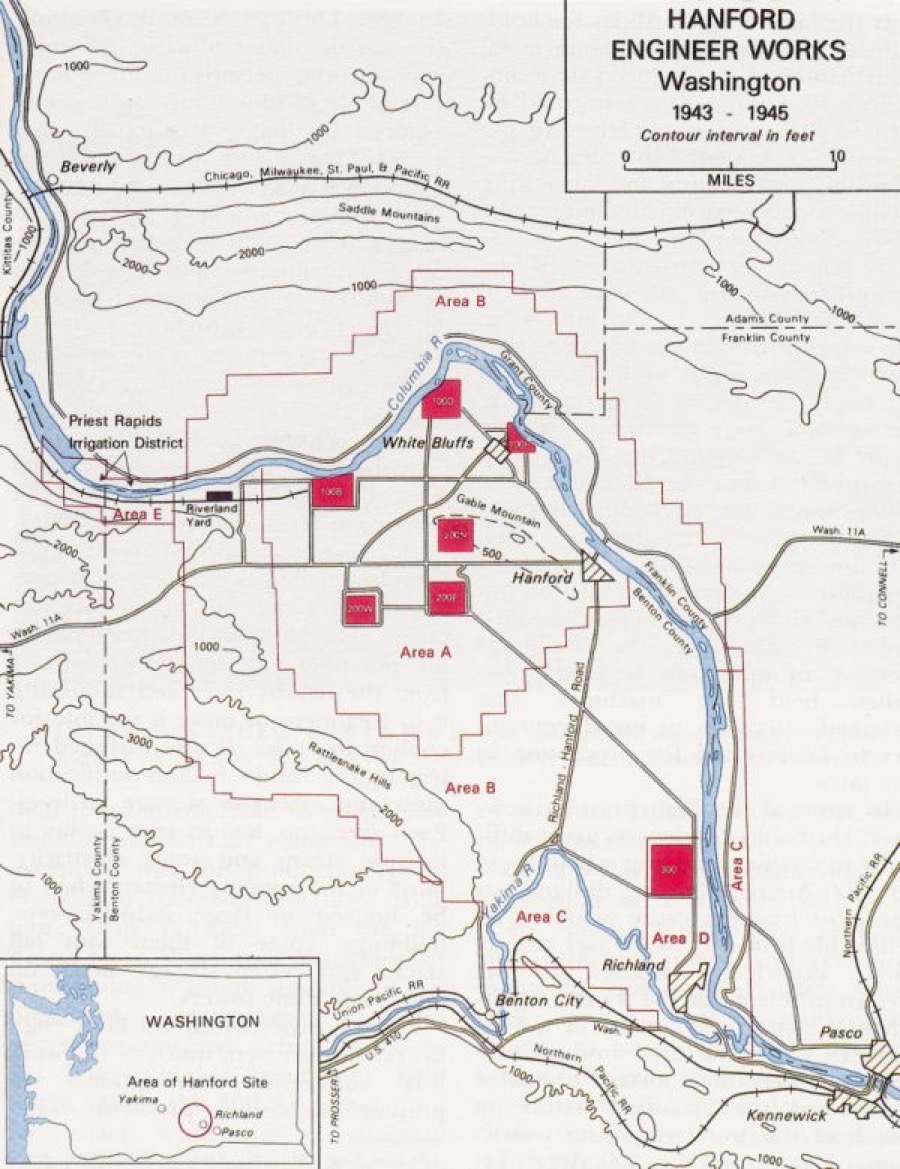
December 16, 1942, found Colonel Franklin Thomson Matthias of Groves’s staff and two Du Pont engineers headed for the Pacific Northwest and southern California to investigate possible production sites. Of the possible sites available, none had a better combination of isolation, long construction season, and abundant water for hydroelectric power than those found along the Columbia and Colorado Rivers. After viewing six locations in Washington, Oregon, and California, the group agreed that the area around Hanford, Washington, best met the criteria established by the Met Lab scientists and Du Pont engineers. The Grand Coulee and Bonneville Dams offered substantial hydroelectric power, while the flat but rocky terrain provided excellent support for the huge plutonium production buildings. The ample site of nearly one-half million acres was far enough inland to meet security requirements, while existing transportation facilities could quickly be improved and labour was readily available. Pleased with the committee’s unanimous report, Groves accepted its recommendation and authorised the establishment of the Hanford Engineer Works, codenamed Site W.

Aerial View of Hanford Community
Not only did they need considerable space for 6 atomic piles, 3 separation plants, production facilities, laboratories, and workers village, but they needed 100,000 kilowatts of continuous power and 25,000 gallons of (soft) water per minute for cooling. Finally the estimated cost for acquiring the entire site (670 square miles) was slightly over $5 million.
The X-10 Complex at Oak Ridge
The fall 1942 planning sessions at the Met Lab led to the decision to build a second Fermi pile at Argonne as soon as his experiments on the first were completed and to proceed on the design of the 'Mae West' helium-cooled unit. When Du Pont engineers assessed the Met Lab’s plans in the late fall, they agreed that helium should be given first priority. They placed heavy-water second and urged an all out effort to produce more of this highly effective moderator. Bismuth and water were ranked third and fourth in Du Pont’s analysis.
Priorities changed when Fermi’s calculations demonstrated a higher value for ƙ than anyone had anticipated. Met Lab scientists concluded that a water-cooled pile was now feasible, while Du Pont shifted its interest to air cooling. Since a helium-cooled unit shared important design characteristics with an air-cooled one, Greenewalt thought that an air-cooled semi-works at Oak Ridge would contribute significantly to designing the full-scale facilities at Hanford. Du Pont established the general specifications for the air-cooled semi-works and chemical separation facilities in early 1943. A massive graphite block, protected by several feet of concrete, would contain hundreds of horizontal channels filled with uranium slugs surrounded by cooling air. New slugs would be pushed into the channels on the face of the pile, forcing irradiated ones at the rear to fall into an underwater bucket. The buckets of irradiated slugs would be left to decay for several weeks, then be moved by an underground canal into the chemical separation facility where the plutonium would be extracted with remote control equipment.
The British designed Magnox, first built at Calder Hall, was built principally to produce plutonium for nuclear weapons. They were pressurised, carbon dioxide cooled, graphite moderated reactors using natural uranium (i.e. unenriched) as fuel and magnox (magnesium-aluminium) alloy as fuel cladding.
Met Lab activities focused on designing a water-cooled pile for the full-scale plutonium plant. Taking their cue from the Du Pont engineers, who utilised a horizontal design for the air-cooled semi-works, Met Lab scientists abandoned the vertical arrangement with water tanks, which had posed serious engineering difficulties. Instead they proposed to use uranium slugs sealed in aluminium cans inside aluminium tubes. The tubes, laid horizontally through a graphite block, would cool the pile with water injected into each tube. The pile, containing 200 tons of uranium and 1,200 tons of graphite, would need 75,000 gallons of water per minute for cooling.
Greenewalt’s initial response to the water-cooled design was guarded. He worried about pressure problems that might lead to boiling water in individual tubes, corrosion of slugs and tubes, and the 1% margin of safety. But he was even more worried about the proposed helium-cooled model. He feared that the compressors would not be ready in time for Hanford, that the shell could not be made vacuum-tight, and that the pile would be extremely difficult to operate. Du Pont engineers conceded that Greenewalt’s fears were well grounded. Late in February 1943, Greenewalt reluctantly concluded that the Met Lab’s model, while it had its problems, was superior to Du Pont’s own helium cooled design and decided to adopt the water-cooled approach. The Met Lab’s victory in the pile design competition came as its status within the Manhattan Project was changing. Still an exciting place intellectually, the Met Lab occupied a less central place in the bomb project, as Oak Ridge and Hanford rose to prominence. Fermi continued to work on the Stagg Field pile (CP-1), hoping to determine the exact value of ƙ. Subsequent experiments at the Argonne site using CP-2, built with material from CP-1, focussed on neutron capture probabilities, control systems and instrument reliability. Once the production facilities at Oak Ridge and Hanford were underway, however, Met Lab research became increasingly unimportant in the race for the bomb and the scientists found themselves serving primarily as consultants for Du Pont.
While the Met Lab physicists chafed under Du Pont domination, a smoother and quieter relationship existed between the chemists and Du Pont. Seaborg and Cooper continued to work well together, and enough progress was made in the semi-works for the lanthanum fluoride process in late 1942 that Du Pont moved into the plant design stage and converted the semi-works for the bismuth phosphate method. Du Pont pressed for a decision on plutonium extraction methods in late May, 1992. Greenewalt chose bismuth phosphate, though even Seaborg admitted he could find little to distinguish between the two. Greenewalt based his decision on the corrosiveness of lanthanum fluoride and on Seaborg’s guarantee that he could extract at least 50% of the plutonium using bismuth phosphate. Du Pont began constructing the chemical separation pilot plant at Oak Ridge, while Seaborg continued refining the bismuth phosphate method.
It was now Cooper’s job to design the pile as well as the plutonium extraction facilities at Clinton, both complicated engineering tasks made even more difficult by high levels of radiation produced by the process. Not only did Cooper have to oversee the design and fabrication of parts for yet another new Manhattan Project technology, he had to do so with an eye toward planning the Hanford facility. Safety was a major consideration because of the hazards of working with plutonium, which was highly radioactive. Uranium, a much less active element than plutonium, posed far fewer safety problems.
In July 1942 Compton also setup a health division at the Met Lab and put Robert S. Stone in charge. Stone established emission standards and conducted experiments on radiation hazards, providing valuable planning information for the Oak Ridge and Hanford facilities.
Du Pont broke ground for the X-10 complex at Oak Ridge in Feburary 1943. The site would include an air-cooled experimental pile, a pilot chemical separation plant, and support facilities. Cooper produced blueprints for the chemical separation plants in time for construction to begin in March 1943. A series of huge underground concrete cells, the first of which sat under the pile, extended to one story above ground. Aluminium cans containing uranium slugs would drop into the first cell of the chemical separation facility and dissolve and then go through the extraction process. The pile building went up during the spring and summer, a huge concrete shell seven feet thick with hundreds of holes for uranium slug placement. Slugs were to plutonium piles what the barrier was to gaseous-diffusion that is, an obstacle that could shut down the entire process. The Aluminium Company of American (Alcoa) was the only firm left working on a process to enclose uranium-235 in aluminium sheaths, and it was still having problems. Initial production provided mixed results, with many cans failing vacuum tests because of faulty welds.
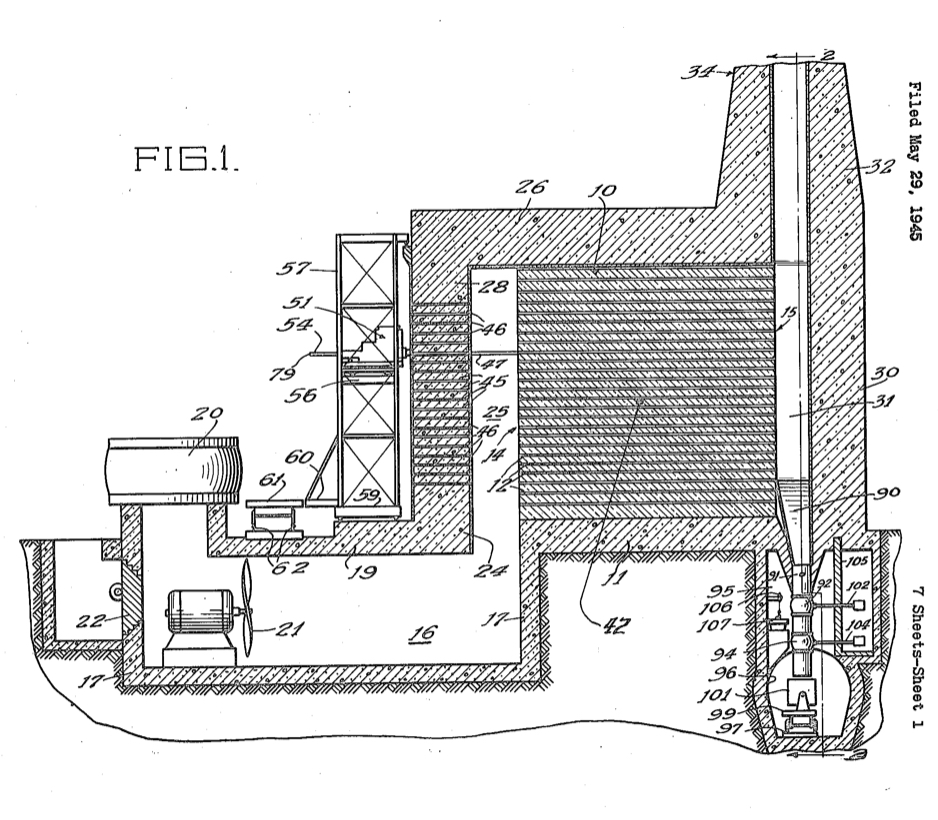
The design shown above is the Fermi patent for an 'Air-Cooled Neutron Reactor'. The basics involve a mass of graphite blocks closely piled into a cube (10) on a concrete foundation (11). The graphite cube is pierced with horizontal air channels (12) of square cross-section, with one of the diagonals vertical. About 1500 channels extend completely through the reactor from the inlet face (14) to the outlet face (15). Adjacent the inlet face the foundation continues downwards to form a floor of an air inlet duct (16) completed by concrete side walls (17) and top (19). This terminates (20) with a fan (21) with access door (22). There is an inlet end shield (24) set away from the inlet face (14) creating an inlet chamber (25) communicating with the air channels (12). At the outlet face (15) there is a shield (30) forming an outlet chamber (31). Below we have a schematic of the X-10 reactor.
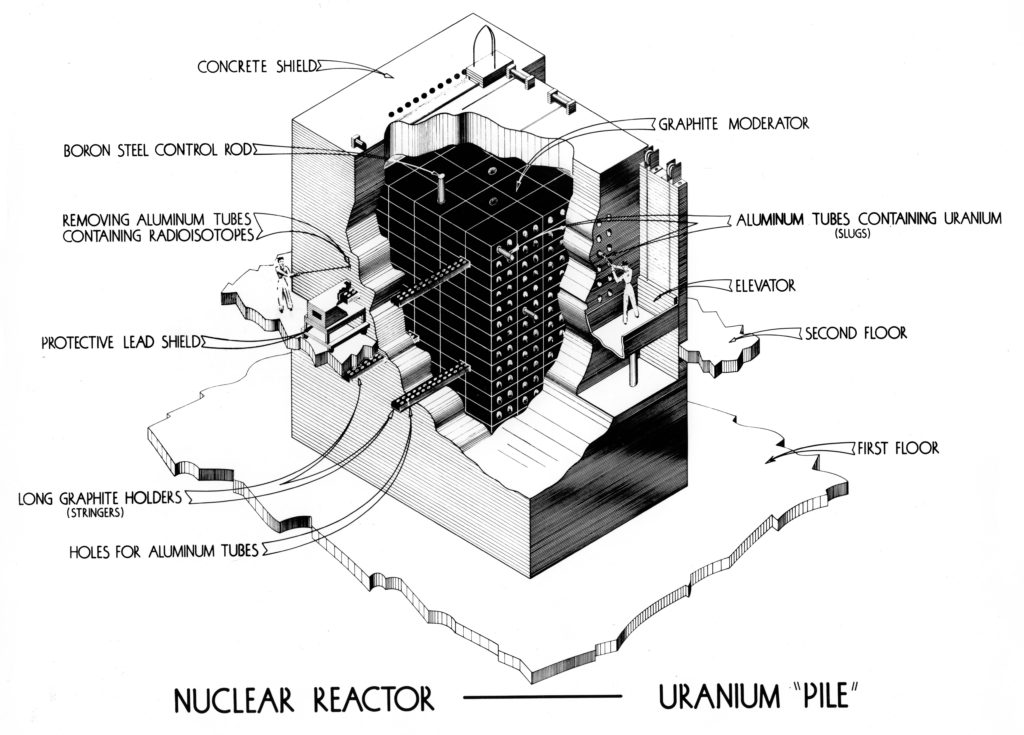
The moment everyone had been waiting for came in late October 1943, when Du Pont completed construction and tests of the X-10 pile at Clinton Engineer Works. After thousands of slugs were loaded, the pile went critical in the early morning of November 4, 1943 and produced plutonium by the end of the month. Criticality was achieved with only half of the channels filled with uranium. During the next several months, Compton gradually raised the power level of the pile and increased its plutonium yield.

Chemical separation techniques using the bismuth phosphate process were so successful that Los Alamos received plutonium samples beginning in the spring 1944. Fission studies of these samples at Los Alamos during summer 1944 heavily influenced bomb design.
Back at Hanford
Colonel Matthias returned to the Hanford area to set up a temporary office on February 22, 1943. His orders had been to purchase half a million acres in and around the Hanford-Pasco-White Bluffs area, a sparsely populated region where sheep ranching and farming were the main economic activities. Many of the area’s landowners rejected initial offers on their land and took the Army to court seeking more acceptable appraisals. Matthias adopted a strategy of settling out of court to save time, time being a more important commodity than money to the Manhattan Project.
Matthias received his assignment in late March, 1943. The three water-cooled piles, designated by the letters B, D, and F, would be built about six miles apart on the south bank of the Columbia River. The four chemical separation plants, built in pairs, would be nearly ten miles south of the piles, while a facility to produce slugs and perform tests would be approximately twenty miles southeast of the separation plants near Richland. Temporary quarters for construction workers would be put up in Hanford, while permanent facilities for other personnel would be located down the road in Richland, safely removed from the production and separation plants.
During summer 1943, Hanford became the Manhattan Project’s newest atomic boomtown. Thousands of workers poured into the town, many of them to leave in discontent. Well situated from a logistical point of view, Hanford was a sea of tents and barracks where workers had little to do and nowhere to go. Du Pont and the Army coordinated efforts to recruit labourers from all over the country for Hanford, but even with a relative labour surplus in the Pacific Northwest, shortages plagued the project. Conditions improved significantly during the second half of the year, with the addition of recreational facilities, higher pay, and better overall service for Hanford’s population, which reached 50,000 by summer 1944. Hanford still resembled the frontier and mining towns once common in the west, but the rate of worker turnover dropped substantially.


Similar to X-10 at Oak Ridge the B reactor (above) was built in Hanford on a much large scale and used water rather than air as a coolant. X-10 was designed with an output of 1 MW (mega-watt), whereas B reactor was designed for 250 MW. It consisted of a core of about 9 meters by 12 meters holding 1,200 tons of graphite cylinders and 2,004 aluminium tubes penetrating horizontally through the entire length of the core. About 100 tons of uranium slugs were packed in roles, and sealed in aluminium cans that went into the tubes. Cooling water from the river was first treated then pumped through the tubes around the uranium cans (I think the long building at the back was the water treatment plant). Water consumption on one reactor core was about the same as for a city of about 300,000 people.

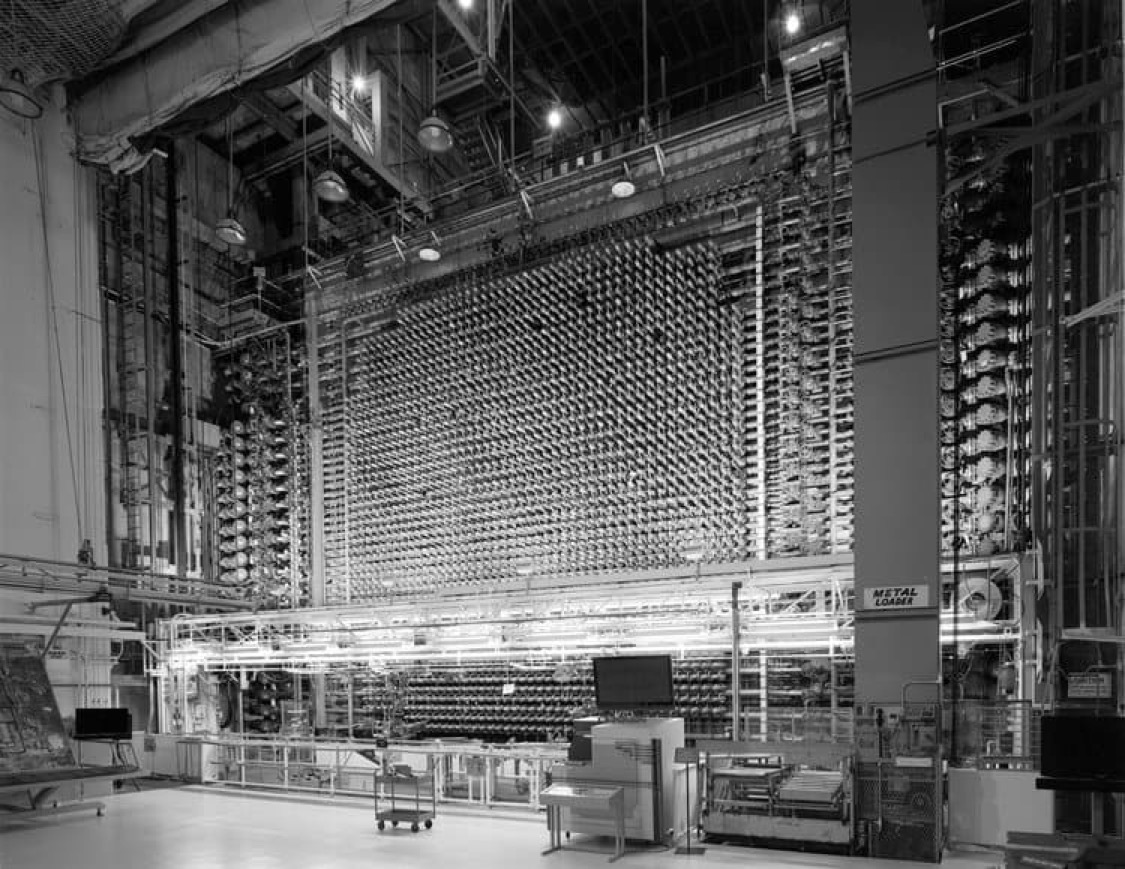
Groundbreaking for the water-cooling plant for the B pile (above we can see workers laying the graphite core for the reactor and the finished reactor face), the western most of the three, took place on August 27, 1944, less than two weeks before Italy’s surrender to the Allies on September 8, 1944. Work on the pile itself began in February 1945, with the base and shield being completed by mid-May 1945. It took another month to place the graphite pile and install the top shield and two more months to wire and pipe the pile and connect it to the various monitoring and control devices.
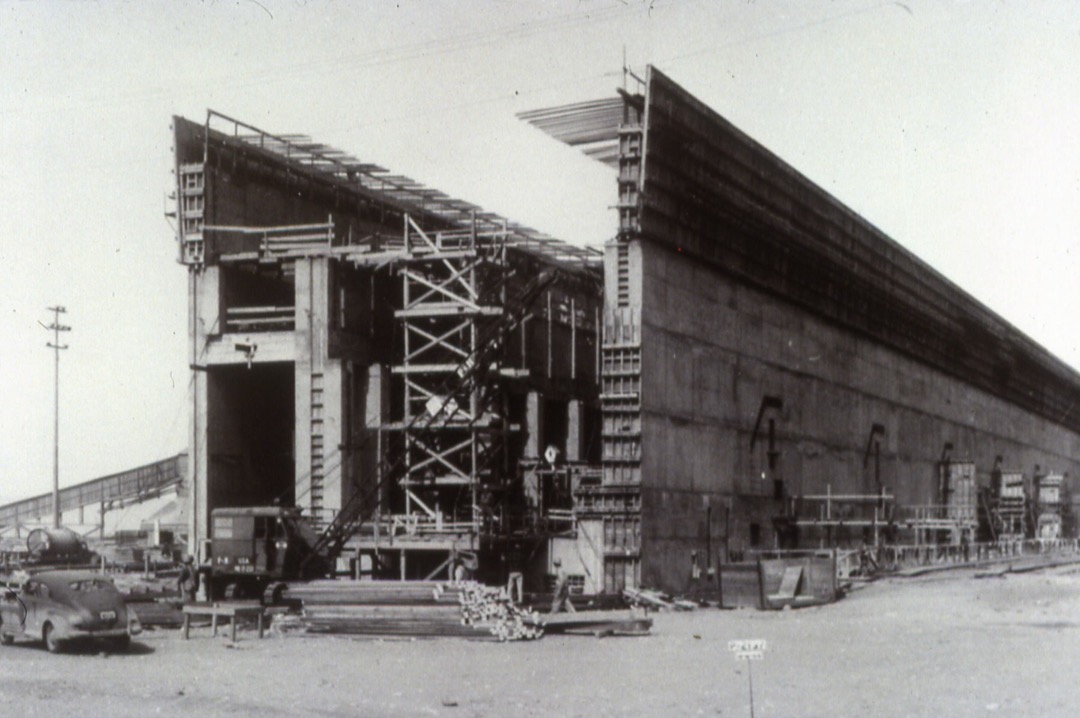

We can see above T plant being built. T and U plants were what might be called first generation chemical separation facilities, and REDOX can be called a second generation process, i.e. the PUREX process.
At Hanford, irradiated uranium slugs would drop into water pools behind the piles and then be moved by remote controlled rail cars to a storage facility five miles away for transportation to their final destination at one of the two chemical separation locations, designated 200-West and 200-East. The T and U plants were located at 200-West, while a single plant, the B unit, made up the 200-East complex (the planned fourth chemical separation plant was not built). The Hanford chemical separation facilities were massive scaled-up versions of those at Oak Ridge, each containing separation and concentration buildings in addition to ventilation (to eliminate radioactive and poisonous gases) and waste storage areas. Labour shortages and the lack of firm blueprints forced Du Pont to stop work on the 200 areas in summer 1943 and concentrate its forces on B pile, with the result that 1943 construction progress on chemical separation was limited to digging two huge holes in the ground.

Here we have the “Queen Mary” structure going up, and one of the finished cells. This was T-plant, and it got its name because it was long and thin like the ocean liner. Today T-plant is no longer operational, and it is now used as a decommissioning and repair facility for treating, verifying and re-packaging waste. T Plant is the only processing canyon at Hanford that remains in operation, although its mission today does not have anything to do with producing plutonium for weapons. The B Plant, S Plant (REDOX), U Plant, and PUREX were the other four processing canyons at Hanford which have long been shut down.
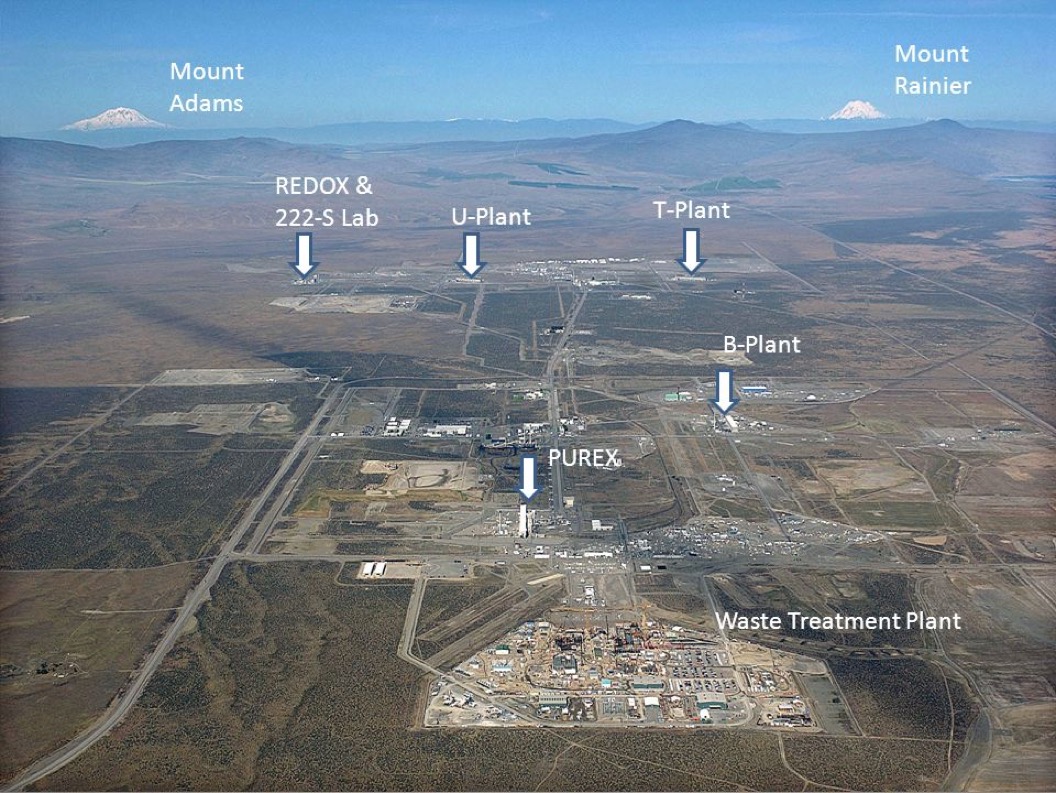
Both 221-T and 221-U, the chemical separation buildings in the 200-West complex, were finished by December 1944. 221-B, the counterpart in 200-East, was completed in spring 1945. Nicknamed Queen Mary’s by the workers who built them, the separation buildings were awesome canyon-like structures 800 feet long, 65 feet wide, and 80 feet high containing forty process pools. The interior had an eerie quality as operators behind thick concrete shielding manipulated remote control equipment by looking through television monitors and periscopes from an upper gallery. Even with massive concrete lids on the process pools, precautions against radiation exposure were necessary and influenced all aspects of plant design.
Construction of the chemical concentration buildings (224-T, -U, and -B) was a less daunting task because relatively little radioactivity was involved, and the work was not started until very late 1944. The 200-West units were finished in early October 1944, the East unit in February 1945. In the Queen Mary’s, bismuth phosphate carried the plutonium through the long succession of process pooIs. The concentration stage was designed to separate the two chemicals. The normal relationship between pilot plant and production plant became evident when the Oak Ridge pilot plant reported that bismuth phosphate was not suitable for the concentration process but that Seaborg’s original choice, lanthanum fluoride, worked quite well. Hanford, accordingly incorporated this suggestion into the concentration facilities. The final step in plutonium extraction was isolation, performed in a more typical laboratory setting with little radiation present. Here Perlman’s earlier research on the peroxide method paid off and was applied to produce pure plutonium nitrate. The nitrate was converted to metal in Los Alamos, New Mexico.
Los Alamos - Project Y
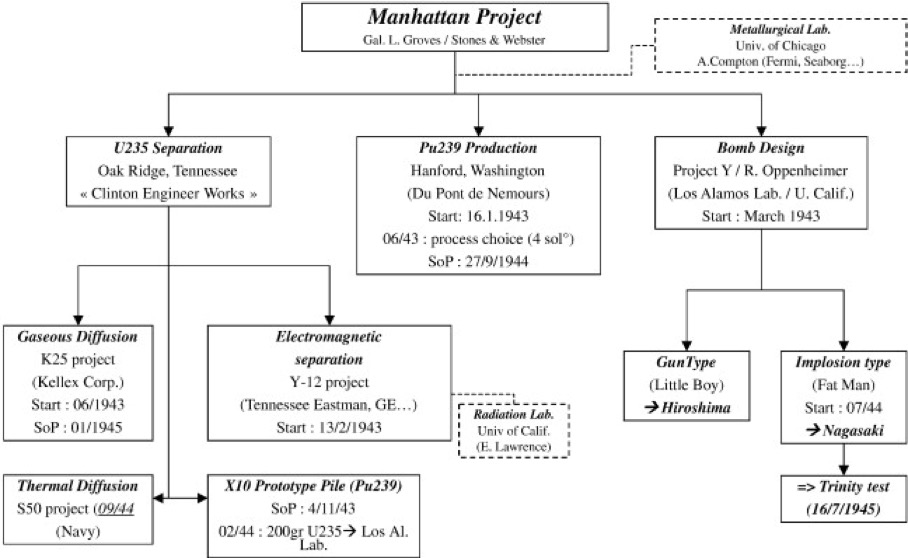
The final link in the Manhattan Project’s far flung network was the Los Alamos Scientific Laboratory in Los Alamos, New Mexico. The laboratory that designed and fabricated the first atomic bombs, code named Project Y, began to take shape in spring 1942 when Conant suggested to Bush that the Office of Scientific and Research Development and the Army form a committee to study bomb development. Bush agreed and forwarded the recommendation to Vice President Wallace, Secretary of War Stimson, and General Marshall (the Top Policy Group). By the time of his appointment in late September 1942, Groves had orders to setup a committee to study military applications of the bomb. Meanwhile, sentiment was growing among the Manhattan scientists that research on the bomb project needed to be better coordinated. Oppenheimer, among others, advocated a central facility where theoretical and experimental work could be conducted according to standard scientific protocols. This would insure accuracy and speed up progress. Oppenheimer suggested that the bomb laboratory operate secretly in an isolated area but allow free exchange of ideas among the scientists on the staff. Groves accepted Oppenheimer’s suggestion and began seeking an appropriate location.
It has been suggested that Groves wanted an American Nobel Prize winner as director of Los Alamos, but Lawrence, Rabi, and Compton were all already involved in critical projects, so Groves turned to Oppenheimer because he had been impressed by the physicist during a meeting on fission research.
Backtracking a bit, in early 1942 there was already a need to start thinking about the weapon. But first they needed to refine the crude estimates of the critical mass and weapon efficiencies, and so the first step was to compile data on basic nuclear reactions, and to coordinate basic experiments on fast-neutron reactions across several research teams. Compton had given this task to Gregory Breit, but with little authority and almost no priority. He knew this task was going be important for the design of a weapon, but few were willing to bend to his needs. So Breit resigned and Compton replaced him by Robert Oppenheimer, and gave him John Henry Manley as an assistant.
Between 1927 and 1963 Breit was four times Associate Editor of the Physical Review. Manley later became a group leader in Los Alamos, then worked for the Atomic Energy Commission, before returning to Los Alamos as assistant director of research.
Oppenheimer brought together a group that quickly realised that there were no major gaps in the theory of fast neutron interactions, but that the lack of quality experimental data made it impossible to do more than suggest ranges for amount of fissionable material needed, the efficiency of the weapon, and its potential destructive effects.
If anything there was a strong feeling that the amount of fissionable material needed had been underestimated. On the other hand the group suggested that an even more powerful reaction might be produced by the thermonuclear fusion of deuterium.
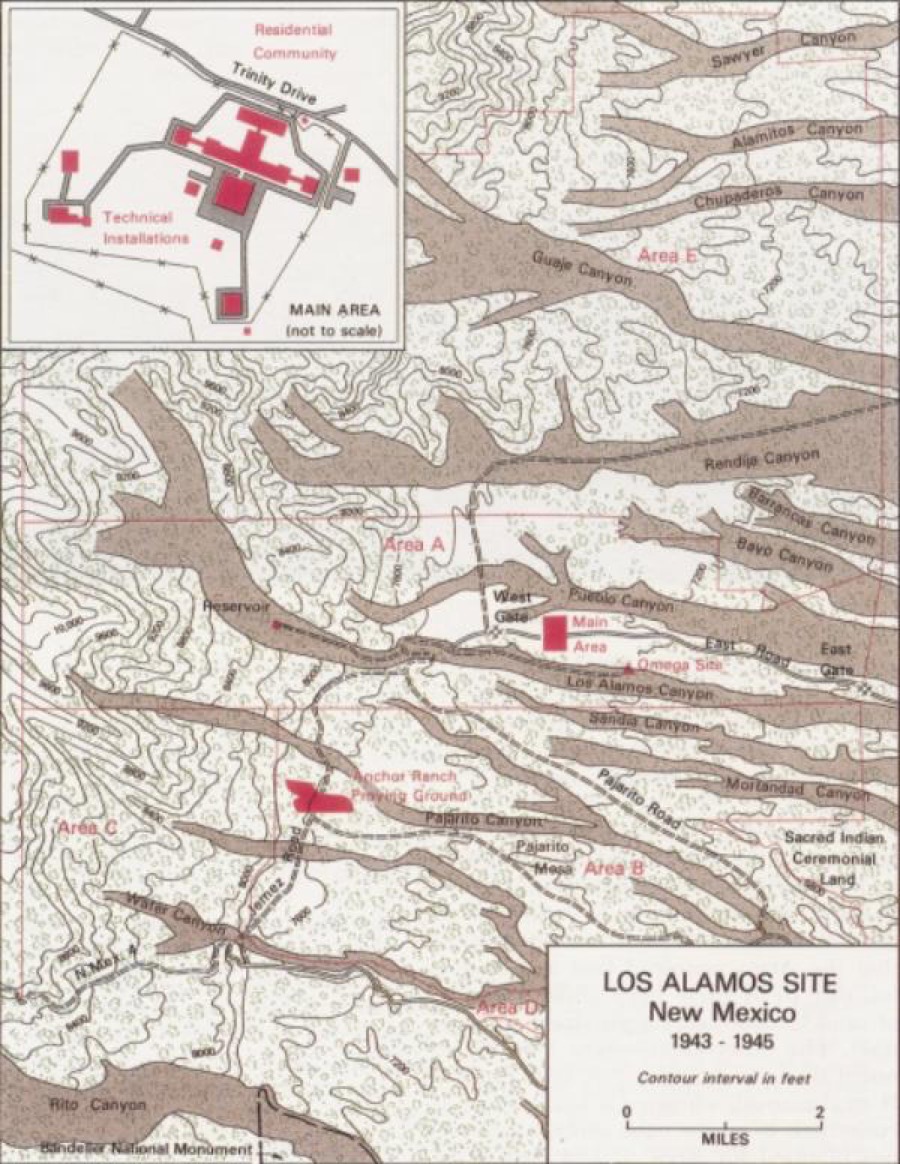
The search for a bomb laboratory site quickly narrowed to two places in northern New Mexico, Jemez Springs and the Los Alamos Boys Ranch School, locations Oppenheimer knew well since he had a ranch nearby in the Pecos Valley of the Sangre de Cristo Mountains. In mid-November, Oppenheimer, Groves, Edwin Mattison McMillan, and Lieutenant Colonel W. H. Dudley visited the two sites and chose Los Alamos. Located on a mesa about thirty miles northwest of Santa Fe, Los Alamos was virtually inaccessible. It would have to be provided with better water and power facilities, but the laboratory community was not expected to be very large. The boys’ school occupying the site was eager to sell, and Groves was equally eager to buy. By the end of 1942 the district engineer in Albuquerque had orders to begin construction, and the University of California had contracted to provide supplies and personnel.

The new site was initially classed as a demolition range, then as Site Y, Project Y, Zia Project, Santa Fe, or finally just Los Alamos. The site was officially activated as a military establishment on April 1, 1943, and was unique in that it was a separate organisation directly responsible to Groves, and run by the University of California.
Oppenheimer, as head of the new laboratory, proved to be an excellent director despite initial concerns about his administrative inexperience, leftist political sympathies, and lack of a Nobel Prize when several scientists he would be directing were prizewinners. Groves worked well with Oppenheimer although the two were fundamentally different in temperament. Groves was a practical-minded military man, brusque and goal oriented. His aide, Colonel Nichols, characterised his heavyset boss as ruthless, egotistical, and confident, “the biggest S.O.B. I have ever worked for. He is most demanding. He is most critical. He is always a driver, never a praiser. He is abrasive and sarcastic”, Nichols admitted, however, that if he had it to do over again, he would once again “pick General Groves [as his boss]” because of his unquestioned ability. Groves demanded that the Manhattan Project scientists spend all their time on the bomb and resisted the temptation, harmless enough in peacetime, to follow lines of research that had no direct applicability to immediate problems.
In contrast to Groves, Oppenheimer was a philosophical man, attracted to Eastern mysticism and of a decidedly theoretical inclination and sensitive nature. A chain-smoker given to long working hours, Oppenheimer appeared almost emaciated. The Groves-Oppenheimer alliance, though not one of intimacy, was marked by mutual respect and was a major factor in the success of the Manhattan Project.
Oppenheimer insisted, with some success, that scientists at Los Alamos remain as much an academic community as possible, and he proved adept at satisfying the emotional and intellectual needs of his highly distinguished staff. Hans Bethe, head of the theoretical division, remembered that nobody else in that laboratory “... even came close to him. In his knowledge. There was human warmth as well. Everybody certainly had the impression that Oppenheimer cared what each particular person was doing. In talking to someone he made it clear that that person’s work was important for the success of the whole project”.
Oppenheimer had a chance to display his persuasive abilities early when he had to convince scientists, many of them already deeply involved in war related research in university laboratories, to join his new organisation.

Complicating his task was the fact that Groves and Oppenheimer planned to operate Los Alamos as a military laboratory. Oppenheimer accepted Groves’s rationale for this arrangement but soon found that scientists objected to working as commissioned officers and feared that the military chain of command was ill suited to scientific decision making. The issue came to a head when Oppenheimer tried to convince Robert F. Bather and Isidor I. Rabi of the Massachusetts Institute of Technology’s Radiation Laboratory to join the Los Alamos team. Neither thought a military environment was conducive to scientific research. At Oppenheimer’s request, Conant and Groves wrote a letter explaining that the secret weapon-related research had presidential authority and was of the utmost national importance.
The letter promised that the laboratory would remain civilian through 1943, when it was believed that the requirements of security would require militarisation through the final stages of the project (in fact, militarisation never took place). Oppenheimer would supervise all scientific work, and the military would maintain the post and provide security.
Oppenheimer spent the first three months of 1943 tirelessly crisscrossing the U.S. in an attempt to put together a first-rate staff, an effort that proved highly successful. Even Bather signed on, though he promised to resign the moment militarisation occurred. Rabi, though he did not move to Los Alamos, became a valuable consultant. As soon as Oppenheimer arrived at Los Alamos in mid-March, recruits began arriving from universities across the United States, including California, Minnesota, Chicago, Princeton, Stanford, Purdue, Columbia, IowaState, and the Massachusetts Institute of Technology, while still others came from the Met Lab and the National Bureau of Standards. Virtually overnight Los Alamos became an ivory tower frontier boomtown, as scientists and their families, along with nuclear physics equipment, including two Van de Graaff’s, a Cockroft-Walton accelerator, and a cyclotron, arrived caravan fashion at the Santa Fe railroad station and then made their way up to the mesa along the single primitive road. It was a most remarkable collection of talent and machinery that settled this remote outpost of the Manhattan Project.
It has been noted that the average age of the scientists in Los Alamos in 1945 was 29 years old.
Theory and the 'Gadget'
Organised by Oppenheimer into specialised research and technical divisions and groups, the Los Alamos scientists divided their efforts between two fundamental tasks, solving the theoretical and experimental problems of a fission bomb, and working out the complex ordnance and engineering problems of weapon design and fabrication. Their concentrated activity over a two-year period, from 1943 to 1945, transformed the laboratory, for all intents and purposes, into a weapon assembly and test plant.
'Gadget' was the code name given to the first bomb tested as part of the Trinity nuclear test.
The initial spartan environment of “the Hill” (which included box lunches and temporary housing) was without doubt a contrast to the comfortable campus settings so familiar to many of the staff. But the laboratory’s work began even as the Corps of Engineers struggled to provide the amenities of civilised life. The properties of uranium were reasonably well understood, those of plutonium less so, and knowledge of fission explosions entirely theoretical. That 2.2 secondary neutrons were produced when uranium-235 fissioned was accepted, but while Seaborg’s team had proven in March 1941 that plutonium underwent neutron induced fission, it was not known yet if plutonium released secondary neutrons during bombardment.

A plutonium pit is the key part of a nuclear warhead. Each pit, slightly warm to the touch, has about 30 parts, which are often coated with nickel or beryllium. Engineered to extraordinary tolerances, the parts fit together like a three-dimensional puzzle. The U.S. lost the capability to produce plutonium pits for its nuclear weapons stockpile in 1989 after a raid by the Federal Bureau of Investigation investigating alleged environmental crimes at the Rocky Flats Plant near Denver, Colorado. At that time, the Rocky Flats Plant was the stockpile plutonium pit production facility for the American nuclear weapons complex. In 1996, DOE officially decided to relocate plutonium pit production to the Los Alamos National Laboratory.
Since then, the U.S. has made at most 11 pits per year. U.S. policy is to maintain existing nuclear weapons. To do this, the Department of Defense (DoD) states that it needs the Department of Energy (DoE), which maintains U.S. nuclear weapons, to produce 50-80 pits per year by 2030. We have to remember that the the Pantex nuclear facility in Amarillo, Texas stores a staggering 15,000 plutonium pits. Meanwhile, Los Alamos planned the construction of the Chemistry and Metallurgy Research Replacement (CMRR), to carry out design and support-work for the pits (replacing the old CMR facility). Wikipedia tells us that reconstruction was in 3 phases, for a total cost of $4-5 billion. Wikipedia also tells us that the first phase was delayed in 2012, other reports say it was cancelled in 2014. The plans changed to the building of an upgraded facility and underground modular plutonium pit facility for $4.3 billion (recently proposed to defer for 5 years). One part of the original plans, the Radiological Laboratory, Utility, and Office Building (RLUOB) was completed, and began radiological operations in Aug. 2014. U.S. Congressional oversight has called Los Alamos plans a “largely-uncoordinated pastiche of plutonium-related line-item construction projects”, with inappropriate 'mission needs', no functional parameters, and major uncertainties about schedule, cost, scope, and overall feasibility.
Critics argue that the new facility is unnecessary for maintaining the nuclear stockpile and may be seen as a threatening development because it allows for the production of new types of nuclear weapons. Opponents to these new developments claim that there are 2,070 deployed weapons, plus 2,600 spare and reserve weapons, plus 2,300 warheads not being maintained, plus about 16,000 surplus pit, of which 5,000 are in strategic reserve.
The theoretical consensus was that chain reactions took place with sufficient speed to produce powerful releases of energy and not simply explosions of the critical mass itself, but only experiments could test the theory. The optimum size of the critical mass remained to be established, as did the optimum shape. When enough data were gathered to establish optimum critical mass, optimum effective mass still had to be determined. That is, it was not enough simply to start a chain reaction in a critical mass, it was necessary to start one in a mass that would release the greatest possible amount of energy before it was destroyed in the explosion.
In addition to calculations on uranium and plutonium fission, chain reactions, and critical and effective masses, work needed to be done on the ordnance aspects of the bomb, or 'gadget' as it came to be known. Two subcritical masses of fissionable material would have to come together to form a supercritical mass for an explosion to occur.
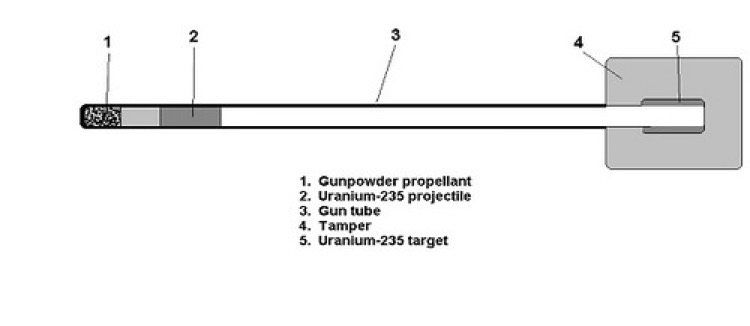
Furthermore, they had to come together in a precise manner and at high speed. Measures also had to be taken to insure that the highly unstable subcritical masses did not pre-detonate because of spontaneously emitted neutrons or neutrons produced by alpha particles reacting with lightweight impurities. The chances of pre-detonation could be reduced by purification of the fissionable material and by using a high-speed firing system capable of achieving velocities of 3,000 feet per second. A conventional artillery method, of firing one subcritical mass into the other was under consideration for uranium-235, but this method would work for plutonium only if absolute purification of plutonium could be achieved (this design was called the gun-type).
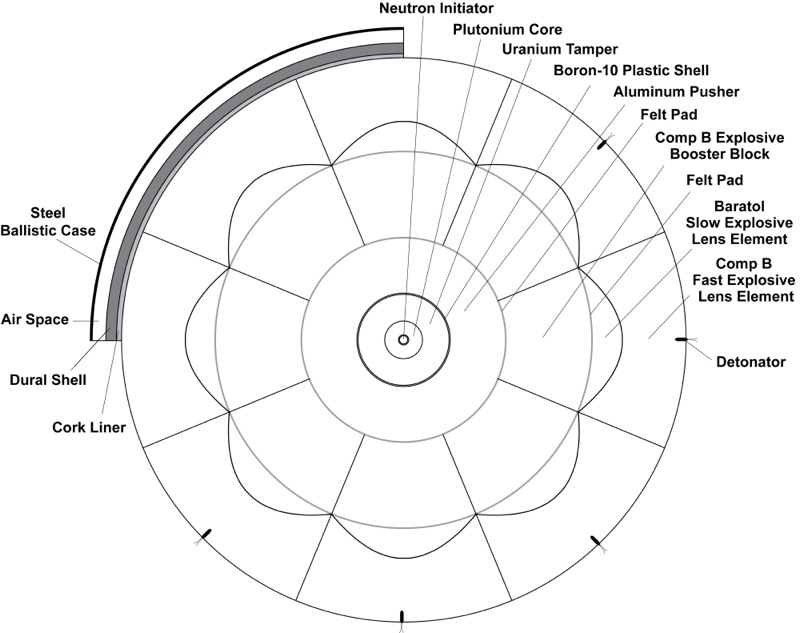
Bomb designers, unable to solve the purification problem, turned to the relatively unknown implosion method for plutonium. With implosion, symmetrical shockwaves directed inward would compress a subcritical mass of plutonium packed in a nickel casing (tamper), releasing neutrons and causing a chain reaction.
Always in the background loomed the hydrogen bomb, a thermonuclear device considerably more powerful than either a uranium or plutonium device but one that needed a nuclear fission bomb as a detonator. Research on the hydrogen bomb, or 'Super', was always a distant second in priority at Los Alamos, but Oppenheimer concluded that it was too important to ignore. After considerable thought, he gave Teller permission to devote himself to the 'Super'. To make up for Teller’s absence, Rudolf Peierls, one of a group of British scientists who reinforced the Los Alamos staff at the beginning of 1944, was added to Bethe’s theory group in mid-1944. Another member of the British contingent was the Soviet agent Klaus Fuchs, who had been passing nuclear information to the Russians since 1942 and continued doing so until 1949 when he was caught and convicted of espionage (and subsequently exchanged).
The first few months at Los Alamos were occupied with briefings on nuclear physics for the technical staff and with planning research priorities and organising the laboratory. Groves called once again on Warren Lewis to head a committee, this time to evaluate the Los Alamos program. The committee’s recommendations resulted in the coordinated effort envisioned by those who advocated a unified laboratory for bomb research. Fermi took control of critical mass experiments and standardisation of measurement techniques. Plutonium purification work, begun at the Met Lab, became high priority at Los Alamos, and increased attention was paid to metallurgy. The committee also recommended that an engineering division be organised to collaborate with physicists on bomb design and fabrication. The laboratory was thus organised into four divisions: theoretical (Hans A. Bethe); experimental physics (Robert F. Bather); chemistry and metallurgy (Joseph William Kennedy); and ordnance (Navy Captain William S. “Deke” Parsons). Like other Manhattan Project installations, Los Alamos soon began to expand beyond initial expectations. As director, Oppenheimer shouldered burdens both large and small, including numerous mundane matters such as living quarters, mail censorship, salaries, promotions, and other 'quality of life' issues inevitable in an intellectual pressure-cooker with few social amenities. Oppenheimer relied on a group of advisers to help him keep the 'big picture' in focus, while a committee made up of Los Alamos group leaders provided day-today communications between divisions.
Los Alamos Makes Progress
Early experiments on both uranium and plutonium provided welcome results. Uranium emitted neutrons in less than a billionth of a second, i.e. just enough time, in the world of nuclear physics, for an efficient explosion. Emilio Segrè later provided an additional cushion with his discovery in December 1943 that, if cosmic rays were eliminated, the subcritical uranium masses would not have to be brought together as quickly as previously thought, nor would the uranium have to be as pure. Muzzle velocity for the scaled down artillery piece could be lower, and a gun-type weapon could be shorter and lighter.
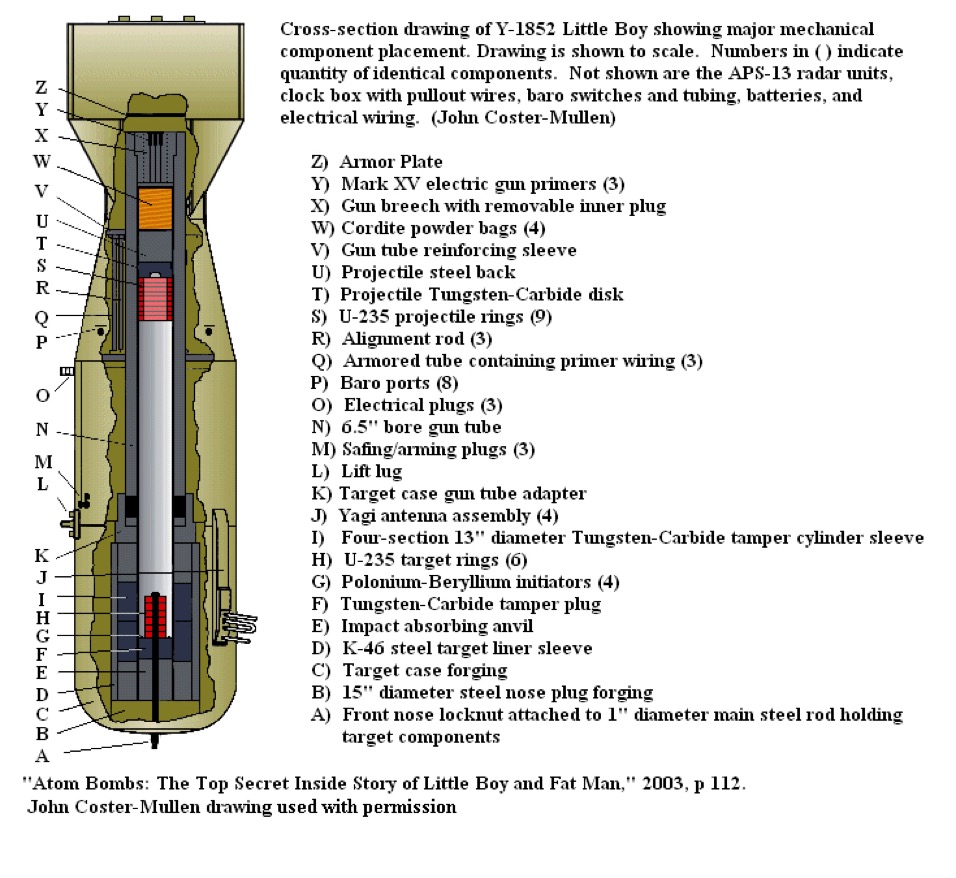
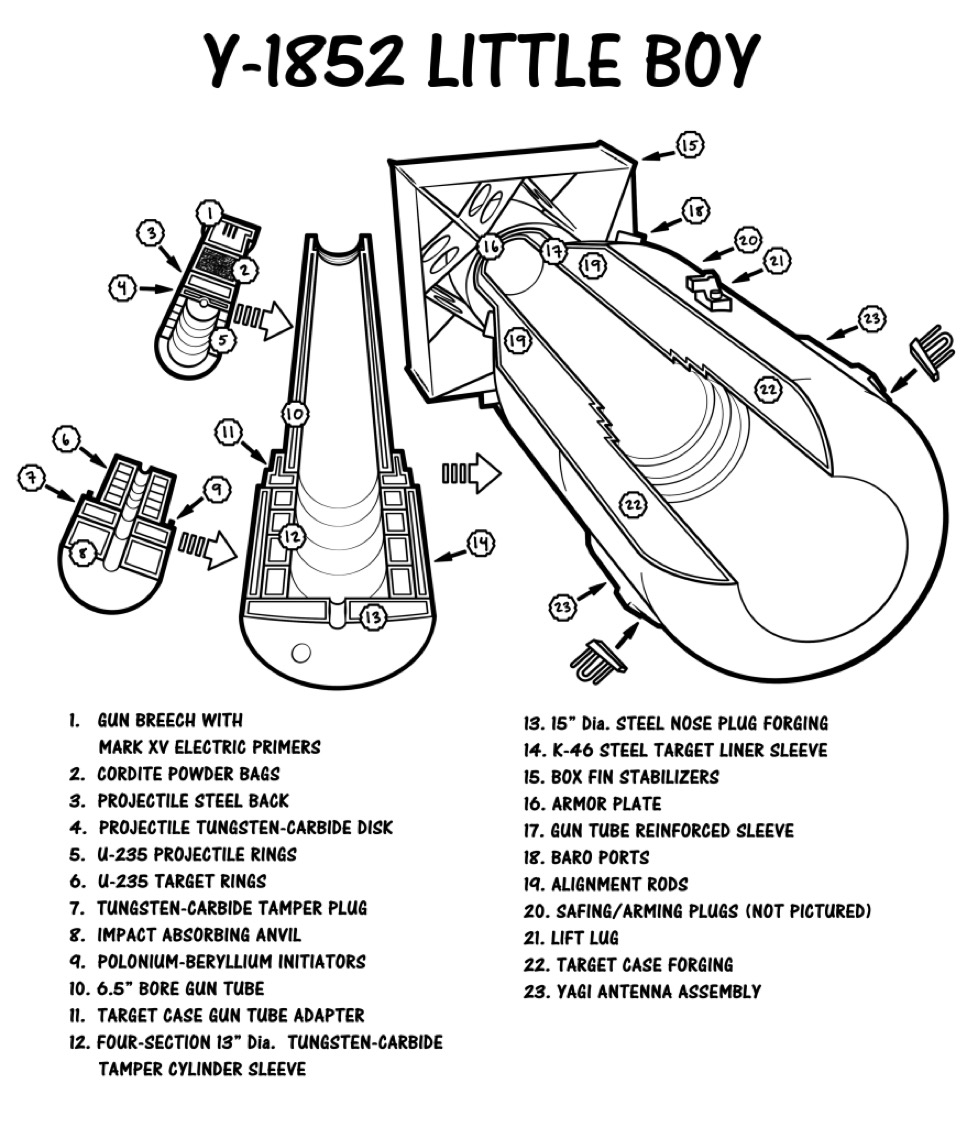
Segrè’s tests on the first samples of plutonium demonstrated that plutonium emitted even more neutrons than uranium due to the spontaneous fission of plutonium-240. Both theory and experimental data now agreed that a bomb using either element would detonate if it could be designed and fabricated into the correct size and shape. But many details remained to be worked out, including calculations to determine how much uranium-235 or plutonium would be needed for an explosive device.
So the physicists gathered considerable data on the effect of cosmic rays on fissioning, on measurement of nuclear cross-sections, on scattering phenomena, and on other aspects of the fission process that related to bomb specifications and efficiency. With this data they were able to calculate by the summer of 1944 that the destructive effect of either an implosion- or gun-type bomb would justify the effort required to fabricate it.
Bather’s engineering division patiently generated the essential cross-sectional measurements needed to calculate critical and efficient mass. The same group utilised particle accelerators to produce the large numbers of neutrons needed for its cross-sectional experiments. Bather’s group also compiled data that helped identify tamper materials (a neutron reflector) that would most effectively push neutrons back to the core and enhance the efficiency of the explosion. Despite Los Alamos’s postwar reputation as a mysterious retreat where brilliant scientists performed miracles of nuclear physics, much of the work that led to the atomic bombs was extremely tedious.
A 'tamper' is an optional layer of dense material surrounding the fissile material. Due to its inertia it delays the expansion of the reacting material, increasing the efficiency of the weapon. Often the same layer serves both as tamper and as neutron reflector.
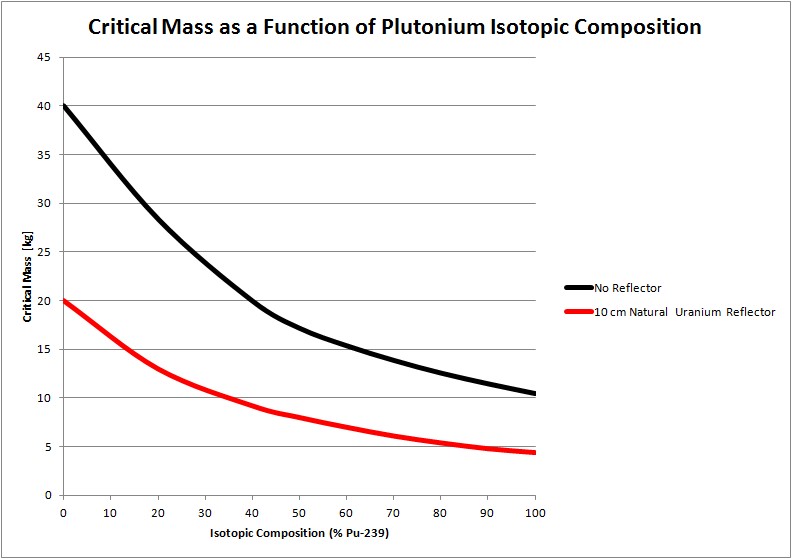
The chemists’ job was to purify the uranium-235 and plutonium, reduce them to metals, and process the tamper material. Only highly purified uranium and plutonium would be safe from pre-detonation. Fortunately purification standards for uranium were relatively modest, and the chemical division was able to focus its effort on the lesser known plutonium and make substantial progress on a multi-step precipitation process by summer 1944. The metallurgy division had to turn the purified uranium-235 and plutonium into metal. Here, too, significant progress was made by summer of 1944, as the metallurgists adapted a stationary-bomb technique initially developed at Iowa State University. Parsons, in charge of ordnance engineering, directed his staff to design two artillery pieces of relatively standard specifications except for their extremely light barrels. One for a uranium weapon and one for a plutonium bomb. The weapons needed to achieve high velocities, but they would not have to be durable since they would only be fired once. Here again early efforts centred on the more problematic plutonium weapon, which required a higher velocity due to its higher risk of pre-detonation. Two plutonium guns arrived in March 1944 and were field-tested successfully. In the same month, two uranium guns were ordered.
Since no one could yet answer the question: How much fissionable material would be needed for an effective weapon? One way to increase the efficiency of a fission bomb was to achieve maximum purity in the active materials. Hence, a major program of the laboratory's chemistry and metallurgy division was to improve the methods for purifying uranium-235 and plutonium-239. Because purity requirements for uranium were about one-third less than those for plutonium and because, until early 1944, there was not enough plutonium-239 available to permit effective work on its purification, the chemists experimented with uranium but with the purpose of developing techniques that might also be used with plutonium. When sufficient amounts of plutonium-239 arrived from the Clinton pile, the chemists developed both wet and dry purification processes. Subsequently, they employed the more satisfactory wet process in the final purification of most the plutonium for the bomb.
Before uranium-235 or plutonium-239 could be used in a fission bomb, they had to be converted into metal of the proper configuration and purity. Metallurgists at Los Alamos faced a number of problems in making uranium or plutonium metal of the desired quality, including the tendency of uranium to catch fire during processing and the difficulty of handling the highly reactive and poisonous plutonium. For forming uranium into metal, they experimented with electrolytic and centrifuge processes but finally settled upon a modification of the stationary bomb method, devised earlier at Iowa State.
For plutonium, the metallurgists were as handicapped as the chemists, with only microscopic quantities available. Fortunately, many of the methods they developed for uranium proved adaptable to plutonium. Again like the chemists, the metallurgists had to devote considerable effort to devising improved recovery methods so that virtually none of the precious metal would be lost in processing it for use in a weapon.
Early Implosion Work
Parsons assigned implosion studies a low priority and placed the emphasis on the more familiar artillery (gun) method. Consequently, Seth Henry Neddermeyer performed his early implosion tests in relative obscurity. Neddermeyer found it difficult to achieve symmetrical implosions at the low velocities he had achieved. When the Princeton mathematician John von Neumann, a Hungarian refugee, visited Los Alamos late in 1943, he suggested that high-speed assembly and high velocities would prevent pre-detonation and achieve more symmetrical explosions. A relatively small, subcritical mass could be placed under so much pressure by a symmetrical implosion that an efficient detonation would occur.
Less critical material would now be required, bombs could be ready earlier, and extreme purification of plutonium would be unnecessary. Von Neumann’s theories excited Oppenheimer, who assigned Parsons’s deputy, George Bogdanovich Kistiakowsky, the task of perfecting implosion techniques. Because Parsons and Neddermeyer did not get along, it was Kistiakowsky who worked with the scientists on the implosion project. While experiments on implosion and explosion continued, Parsons directed much of his effort toward developing bomb hardware, including arming and wiring mechanisms and fusing devices. Working with the Army Air Force, Parsons’s group developed two bomb models by March 1944 and began testing them with B-29’s. 'Thin Man', named for President Roosevelt, utilised the plutonium gun design, while 'Fat Man', named after Winston Churchill, was an implosion prototype. (Segrè’s lighter, smaller uranium 'gadget' became 'Little Boy', 'Thin Man’s' younger brother).
It all dates back to a meeting in April 1943, where the gun approach appeared to provide the surest path to an atomic bomb. It was for this reason that Groves recruited Parsons to head the gun assembly research and development. It was on July 4, 1943, whilst Parsons was absent, that some of his staff conducted a successful proof-of-principle implosion experiment, thus keeping the implosion option alive.
Primarily because of the undeveloped state of the art, interest in implosion research for a time ranked second to gun assembly research. Since April 1943, physicist Seth H. Neddermeyer from the California Institute of Technology had been conducting laboratory experiments with high explosives, designed to test the feasibility of the implosion principle. Neddermeyer's project had definitely remained a 'dark horse' in the race for completion of a workable atomic device.
But all of this changed with the arrival of John von Neumann in midsummer 1943. The widely respected Hungarian-born mathematician from the Institute for Advanced Study at Princeton had been carrying out work on shock waves for the NDRC. Applying knowledge of explosives gained in his work with shaped charges, he theorised the likely effects of increasing the velocity of converging focused active material in the implosion bomb. His calculations convinced him that if the mechanical problems of achieving higher velocity could be solved, an implosion bomb would attain criticality using less active material of a considerably lower level of purity than hitherto believed possible.
If he were correct, implosion offered a means to save precious months in developing a weapon, provided, of course, that ways could be devised to avoid pre-detonation and achieve symmetry in the imploding shock wave inside the bomb.
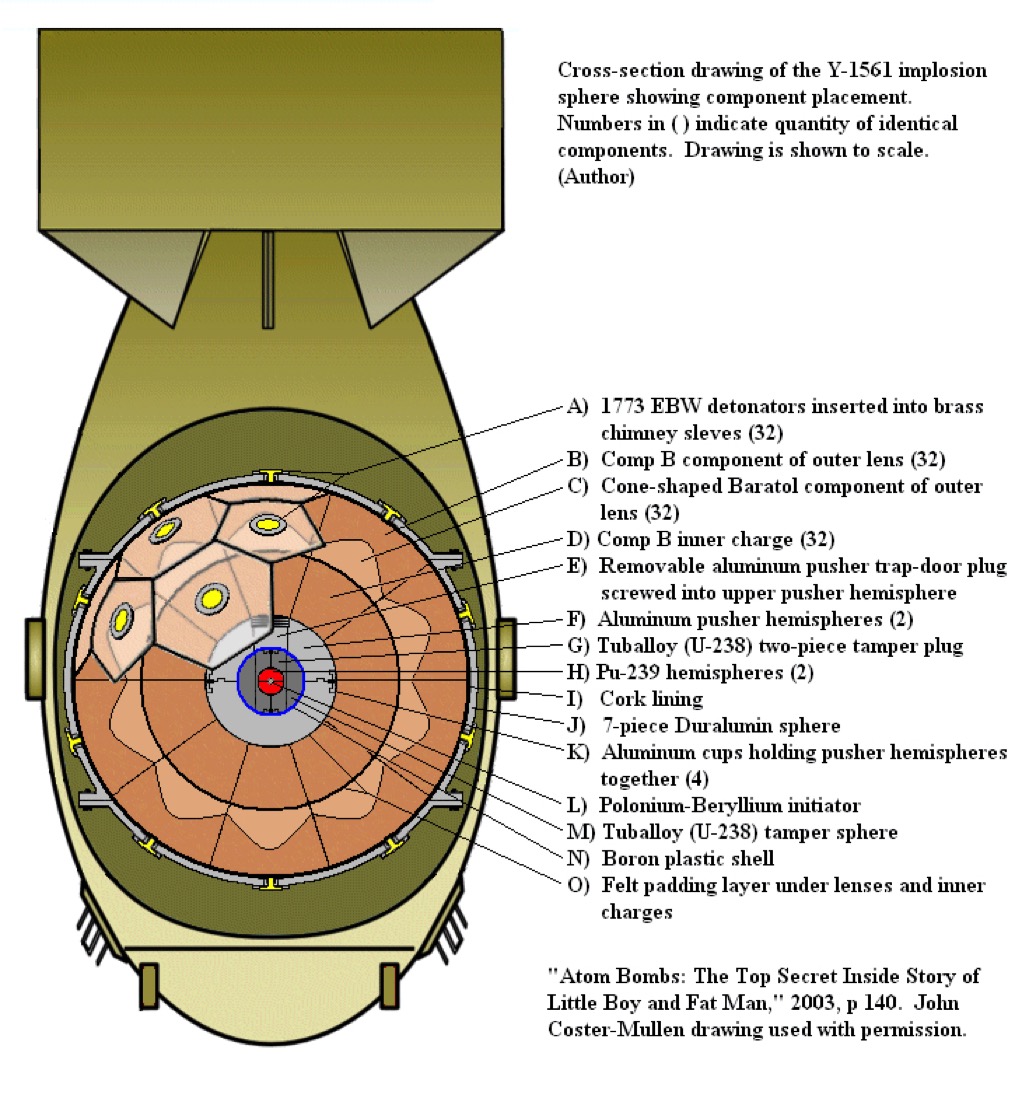
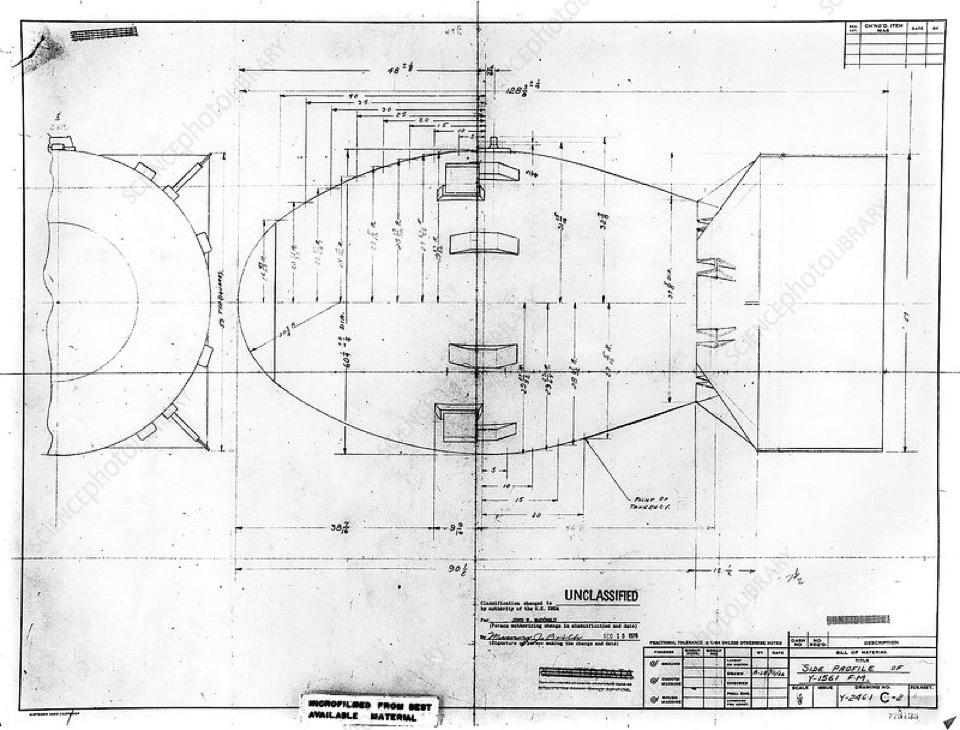
By early fall 1943 Oppenheimer, Groves, Conant, and the other project leaders were re-evaluating implosion. Groves conferred with George B. Kistiakowsky, the distinguished Harvard chemist who was an expert on explosives, and with Oppenheimer and members of the laboratory's implosion study group. This led to a decision by Oppenheimer and the laboratory's governing board to expand the implosion program immediately, beginning with construction of an on-site plant for casting and trimming test components and installation of the unusual facilities required for testing implosion devices.
In early November 1943, Groves and Conant outlined the advantages of implosion to the Military Policy Committee. The following February, the committee informed the President that "there is a chance, and a fair one, if a process involving the use of a minimum amount of material proves feasible, that the first bomb can be produced in the late fall of 1944".
Elimination of 'Thin Man'
'Thin Man' was eliminated four months later because of the plutonium-240 contamination problem. Seaborg had warned that when plutonium-239 was irradiated for a length of time it was likely to pick up an additional neutron, transforming it into plutonium-240 and increasing the danger of pre-detonation (the bullet and target in the plutonium weapon would melt before coming together). Measurements taken at Clinton confined the presence of plutonium-240 in the plutonium produced in the experimental pile. On July 17, 1944, the difficult decision was made to cease work on the plutonium gun method. Plutonium could be used only in an implosion device, but in summer 1944 an implosion weapon looked like a long shot.
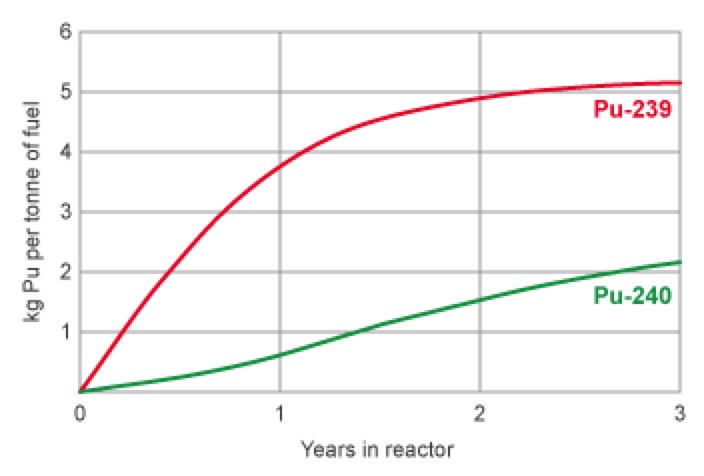
So in July 1944, Los Alamos scientists discovered the disquieting new data on the plutonium that would later be produced in the Hanford piles. The composition of its neutron background would cause pre-detonation in the plutonium gun. Project scientists had known for some time that in the process of irradiating uranium in the pile some of the plutonium-239 was likely to pick up an extra neutron, forming plutonium-240. When plutonium from the Clinton pilot pile became available in the spring of 1944, the radioactivity group at Los Alamos ran a series of tests that confirmed the presence of plutonium-240 and indicated it would be present in far larger amounts in plutonium from the Hanford piles. Hence, the neutron background of the active material for the bombs would be several hundred times greater than was permissible. While the plutonium-240 could be separated from the plutonium-239 by the electro-magnetic process, construction of a plant to do so would delay production of a plutonium weapon for many months.

'Thin Man' plutonium gun test casings at Wendover Army Air Field, as part of Project Alberta in the Manhattan Project. 'Fat Man' casings can be seen behind them.
One report mentions that in spring 1940, an experiment conducted by a small group of graduate students near the location of Ashley Pond’s long-abandoned Pajarito Club, discovered an isotopic impurity in plutonium, one that could not be removed. If plutonium was used in a gun assembled weapon, this impurity would cause a premature explosion, spontaneous fission would cause a pre-detonation. Without an alternative assembly method, plutonium could not be used at all for an atomic bomb.
Ashley Pond was a Detroit businessman who took an option out on the land in 1913, in order to build a private club (resort or 'dude' ranch) for the wealthy. It was called The Pajarito Club after the Pajarito plateau, but it had already failed by 1916. Ashley Pond went on to found of the Los Alamos Ranch School in 1917.
Oppenheimer informed Conant of the plutonium-240 problem in early July 1944, and it was decided that the pre-detonation threat posed by plutonium-240 made the use of plutonium in the gun-type bomb impracticable and work on this system should be suspended immediately. With this decision, even greater urgency was placed on the development of a workable implosion weapon, in which the plutonium-240, because of the higher velocities involved, would be unlikely to cause pre-detonation.
Abandonment of the plutonium gun project eliminated a shortcut to the bomb. This necessitated a revision of the estimates of weapon delivery Bush had given the President in 1943. The new timetable, presented to General Marshall by Groves on August 7, 1944, two months after the Allied invasion of France began at Normandy on June 6, 1944, promised small implosion weapons of uranium or plutonium in the second quarter of 1945 if experiments proved satisfactory. More certain was the delivery of a uranium gun bomb by August 1, 1945, and the delivery of one or two more by the end of that year.
Abandonment of the plutonium gun compelled General Groves to revise his predictions on when an atomic weapon would be ready for employment against the enemy. In a progress report to General Marshall in early August 1944, he presented a revised timetable of weapon production: five to eleven implosion bombs in the period from March through June 1945, with an additional twenty to forty implosion bombs of the same size by the end of the year. He cautioned, however, that this schedule would not apply "if experiments yet to be conducted with an implosion type bomb do not fulfil expectations and we are required to rely on the gun type alone" and suggested that, if this delay should occur, the first bomb would not be ready until August 1, 1945, with one or two more by the year's end. In Groves's opinion, any delay virtually guaranteed that the bomb would not be used against Germany, which by the late summer of 1944 appeared likely to be defeated within a few months. And to many, even the bomb's use against Japan seemed doubtful.
Nuclear Ordnance
Initially the first priority for the Los Alamos’s ordnance division was design and fabrication of a plutonium-projectile gun. This gun type posed more problems than a uranium gun, because of plutonium-239's higher propensity to pre-detonation, but the division's theory that a gun with sufficient muzzle velocity to avoid pre-detonation with plutonium-239 was certain to be suitable for uranium-235 justified the concentration of effort. Using standard ordnance and interior ballistics data obtained from the National Defense Research Committee (NDRC), the ordnance division had its design engineers complete the drawings for a high-velocity gun and, with subsequent approval from the Navy's Bureau of Ordnance, ordered forgings for two guns from the Naval Gun Factory in Washington, D.C. In the meantime, while the guns were being manufactured, Captain Parsons arranged for construction of the Anchor Ranch Proving Ground, some 8 miles east of the central laboratory facilities, where, by September 1943, the division's proving ground group began testing and perfecting gun performance techniques.
Engineers finally established the exact specifications of a low-velocity gun, to be used with uranium-235. Hence, because these specifications were considerably less stringent than previously anticipated for a uranium-235 gun, the engineers were able to reduce the original muzzle velocity requirements. This achievement made it possible for the division to place in March 1944 an order with the Naval Gun Factory for three of these uranium guns, which was much earlier than expected and just days after the factory had delivered the first two plutonium prototypes to Los Alamos (which later proved to be useless due the contamination problem).

Here we see 'Little Boy' in the bomb pit on Tinian Island, and being lifted into Enola Gay. 
The gun-type model, the 'Thin Man', was about 10 feet in length, with a varying diameter of 1.5 to 2.5 feet, and had an estimated weight (when loaded) of 5 tons. The implosion-type model, the 'Fat Man', was almost as long (9 feet) but thicker, tapering down from a hemispherical nose measuring 5 feet in diameter to a tail of about 3 feet, and had an estimated weight (when loaded) of 6 tons. Captain Parsons had models constructed at the Applied Physics Laboratory in Silver Spring, Maryland, and tested at the Naval Proving Ground on the Potomac River at Dahlgren, Virginia. The laboratory's delivery group also conducted in-flight tests in a modified B-29, dropping dummy models of both types of bombs, at the Muroc Army Air Field near San Francisco. The ballistic characteristics of 'Thin Man' were satisfactory, but 'Fat Man' displayed serious instability, fortunately soon overcome by a relatively simple modification in the tail assembly.
Over time there was an inevitable shift in emphasis from research and experimentation to engineering, fabrication, and testing. Construction crews, under direction of Major Wilber A. Stevens, completed a number of essential test areas (eventually there would be more than thirty of these). They had built a facility for casting containers for explosive charges at the Anchor Ranch Proving Ground and, less than a mile to the south, were well advanced on a much larger and more elaborately equipped area, designated S (for Sawmill) Site, with a laboratory, shops, powder magazines, and even a dining hall. In addition, Major Stevens's crews had begun work on several outlying sites required especially for testing various implosion devices. Ordnance teams from Los Alamos also assembled and tested bomb components at test sites at Wendover Field (Utah), Inyokern (California), and Alamogordo Army Air Field (New Mexico). For these tests, the laboratory procured normal weapon components and high explosives from a variety of government and private suppliers, however for special parts and materials that were unobtainable, the laboratory itself had to function as an ordnance manufacturing plant. The best illustration of this effort was the major task of converting uranium-235 and plutonium-239 into metal bomb components.
Japan Becomes the Target
Marshall and Groves acknowledged that a German surrender might take place by summer 1945, thus making it probable that Japan would be the target of any atomic bombs ready at that time.
It was still unclear if even the August 1, 1945, deadline could be met. While expenditures reached $100 million per month by mid-1944, the Manhattan Project’s goal of producing weapons for the current war was not assured. Operational problems plagued the Y-12 electro-magnetic facility just coming on line. The K-25 gaseous-diffusion plant threatened to become an expensive white elephant if a suitable barrier could not be fabricated. And the Hanford piles and separation facilities faced an equally serious threat as not enough of the uranium-containing slugs to feed the pile were available. Even assuming that enough uranium or plutonium could be delivered by the production facilities built in such great haste, there was no guarantee that the Los Alamos laboratory would be able to design and fabricate weapons in time. Only the most optimistic in the Manhattan Project would have predicted, as Groves did when he met with Marshall, that a bomb or bombs powerful enough to make a difference in the current war would be ready by August 1, 1945.
During winter 1944-45 there was substantial progress at Oak Ridge, thanks to improved performance in each of the production facilities and Nichols’s work in coordinating a complicated feed schedule that maximised output of enriched uranium by utilising the electro-magnetic, thermal diffusion, and gaseous-diffusion processes in tandem. Nine Alpha and three Beta racetracks were operational and, while not producing up to design potential, were becoming significantly more reliable because of maintenance improvements and chemical refinements introduced by Tennessee Eastman. The S-50 thermal diffusion plant being built by the H. K. Ferguson Company was almost complete and a section of S-50 was producing small amounts of enriched material, and the K-25 gaseous-diffusion plant, complete with barriers, was undergoing final leak tests. By March 1945, Union Carbide had worked out most of the kinks in K-25 and had started recycling uranium hexafluoride through the system. S-50 was finished at the same time that the Y-12 racetracks were demonstrating increased efficiency. The Beta calutrons at the electro-magnetic plant were producing weapon-grade uranium-235 using feed from the modified Alpha racetracks and the small output from the gaseous-diffusion and thermal diffusion facilities. Oak Ridge was now sending enough enriched uranium-235 to Los Alamos to meet experimental needs. To increase production, Grove sponsored an additional gaseous-diffusion plant (K-27) for low-level enrichment and a fourth Beta track for high-level enrichment, both to be completed by February 1946, in time to contribute to the war against Japan, which many thought would not be conclude before summer 1946.
With the abandonment of the plutonium gun bomb in July 1944, planning at Hanford became more complicated. Pile B was almost complete, as was the first chemical separation plant, while Pile D was at the halfway point. Pile F was not yet under construction. If implosion devices using plutonium could be developed at Los Alamos, the three piles would probably produce enough plutonium for the weapons required, but as yet no one was sure of the amount needed.
Excitement mounted at Hanford as the date for pile start-up approached. Fermi placed the first slug in Pile B on September 13, 1944. Final checks on the pile had been uneventful. The scientists could only hope they were accurate, since once the pile was operational the intense radioactivity would make maintenance of many components impossible. Loading slugs and taking measurements took two weeks. From just after midnight until approximately 3:00 a.m. on September 27, 1944, the pile ran without incident at a power level higher than any previous chain reaction (though only at a fraction of design capacity). The operators were elated, but their excitement turned to astonishment when the power level began falling after three hours. It fell continuously until the pile ceased operating entirely on the evening of September 28, 1944. By the next morning the reaction began again, reached the previous day’s level, then dropped.
Xenon Poisoning
Hanford scientists were at a loss to explain the pile’s failure to maintain a chain reaction (this is now called an iodine “pit”). Only the foresight of Du Pont’s engineers made it possible to resolve the crisis. The cause of the strange phenomenon proved to be xenon poisoning. Xenon, a fission product isotope with a mass of 135, was produced as the pile operated. It captured neutrons faster than the pile could produce them, causing a gradual shutdown. With shutdown, the xenon decayed, neutron flow began, and the pile started up again. Fortuitously, despite the objections of some scientists who complained of Du Pont’s excessive caution, the company had installed a large number of extra tubes. This design feature meant that Pile B could be expanded to reach a power level sufficient to overwhelm the xenon poisoning. Success was achieved when the first irradiated slugs were discharged from Pile B on Christmas Day, 1944. The irradiated slugs, after several weeks of storage, went to the chemical separation and concentration facilities. By the end of January 1945, the highly purified plutonium underwent further concentration in the completed chemical isolation building, where remaining impurities were removed successfully. Los Alamos received its first plutonium on February 2, 1945.
Xenon-135 is a fission product of uranium, and is a 'potent' neutron absorber, with a thermal neutron absorption cross-section of about 2.6 x 106 barns. So Xenon-135 is about 4000 times better than uranium-235 (cross-section of 400-600 barns for thermal fission) in absorbing thermal neutrons (essential to the chain reaction). About 6.6% of all fissions in uranium-235 end up producing a xenon-135 fission product (along with the other fission product strontium-99 with a very, very short half-life of 0.2 s). However about 95% of xenon-135 actually comes from the beta-decay of another fission product, iodine-135 (half-life 6.57 hrs), and indirectly from tellurium-135 through iodine-135. Xenon-135 is removed from the reactor by neutron absorption (radiative capture to xenon-136) or decay (half-life 9.1hrs). Samarium-149 is the next most important absorber of thermal neutrons (cross-section of 4.1 x 104 barns).
The so-called “xenon transient” when shutting down a reactor was part of the problem in the Chernobyl disaster.
The Final Push
Oppenheimer acted quickly to maximise the laboratory’s efforts to master implosion. Only if the implosion method could be perfected would the plutonium produced at Hanford come into play. Without either a plutonium gun bomb or implosion weapon, the burden would fall entirely on uranium and the less efficient gun method. Oppenheimer directed a major reorganisation of Los Alamos in July 1944 that prepared the way for the final development of an implosion bomb. Robert Bather took over G Division (G for 'gadget') to experiment with implosion and design a bomb; George Kistiakowsky led X Division (for explosives) in work on the explosive components; Hans Bethe continued to head up theoretical studies; and “Deke” Parsons (or “Deak”) now focused on overall bomb construction and delivery.
Field tests performed with uranium-235 prototypes in late 1944 eased doubts about the artillery method to be employed in the uranium bomb. It was clear that the uranium-235 from Oak Ridge would be used in a gun-type nuclear device to meet the August 1, 1945, deadline Groves had given General Marshall and the Joint Chiefs of Staff. The plutonium produced at such expense and effort at Hanford would not fit into wartime planning unless a breakthrough in implosion technology occurred.
At the same time, Los Alamos shifted from research to development and production. Time was of the essence, though laboratory research had not yet charted a clear path to the final product. Army Air Force training could wait no longer, and in September 1944, at Wendover Field in western Utah, Colonel Paul Warfield Tibbets Jr. began drilling the 393rd Bombardment Squadron, the heart of the 509th Composite Wing, in test drops with 5,500-pound orange dummy bombs, nicknamed 'pumpkins' (nearly 50 of these practice bombs were dropped on Japan). In June 1945, Tibbets and his command moved to Tinian Island in the Marianas, where the U.S. Navy SeaBees (Construction Battalion) had built the world’s largest airport to accommodate Boeing’s new B-29 Superfortress.
Personnel shortages, particularly of physicists, and supply problems complicated Oppenheimer’s task. The procurement system, designed to protect the secrecy of the Los Alamos project, led to frustrating delays and, when combined with persistent late war shortages, proved a constant headache. The lack of contact between the remote laboratory and its supply sources exacerbated the problem, as did the relative lack of experience the academic scientists had with logistical matters.
Groves and Conant were determined not to let mundane problems compromise the bomb effort, and in fall 1944 they made several changes to prevent this possibility. Conant shipped as many scientists as could be spared from Chicago and Oak Ridge to Los Alamos, hired every civilian machinist he could lay his hands on, and arranged for Army enlisted men to supplement the work force (these GI’s were known as SEDS, for Special Engineering Detachment). Hartley Rowe, an experienced industrial engineer, provided help in easing the transition from research to production. Los Alamos also arranged for a rocket research team at the California Institute of Technology to aid in procurement, test fuses, and contribute to component development. These changes kept Los Alamos on track as weapon design reached its final stages.
Freezing Weapon Design
Weapon design for the uranium gun bomb was frozen in February 1945. Confidence in the weapon was high enough that a test prior to combat use was seen as unnecessary. The design for an implosion device was approved in March 1945, with a test of the more problematic plutonium weapon scheduled for July 4, 1945. Oppenheimer shifted the laboratory into high gear and assigned Allison, Bather, and Kistiakowsky to the 'Cowpuncher Committee' to 'ride herd' on the implosion weapon. He placed Kenneth T. Bainbridge in charge of Project Trinity, a new division to oversee the July 1945 test firing. Parsons headed Project Alberta, known as Project A, which had the responsibility for preparing and delivering weapons for combat.
During these critical months much depended upon the ability of the chemists and metallurgists to process the uranium and plutonium into metal and craft them into the correct shape and size. Plutonium posed by far the greater obstacle. It exists in different states, depending upon temperature, and is extremely toxic. Working under intense pressure, the chemists and metallurgists managed to develop precise techniques for processing plutonium just before it arrived in quantity beginning in May 1945.
As a result of progress at Oak Ridge and metallurgical and chemical refinements on plutonium that improved implosion’s chances, the nine months between July 1944 and April 1945 saw the American bomb project progress from doubtful to probable. The August 1, 1945, delivery date for the “Little Boy” uranium bomb certainly appeared more likely than it had when Groves briefed Marshall. There would be no implosion weapons in the first half of 1945 as Groves had hoped, but developments in April 1945 boded well for the scheduled summer test of the 'Fat Man' plutonium bomb. And recent calculations provided by Bethe’s theoretical group gave hope that the yield for the first weapon would be in the vicinity of 5,000 tons of TNT rather than the 1,000-ton estimate provided in fall 1944.
Here the story continues more or less word-for-word from “Manhattan - The Army and the Atomic Bomb”
Trinity - Testing the Atomic Bomb
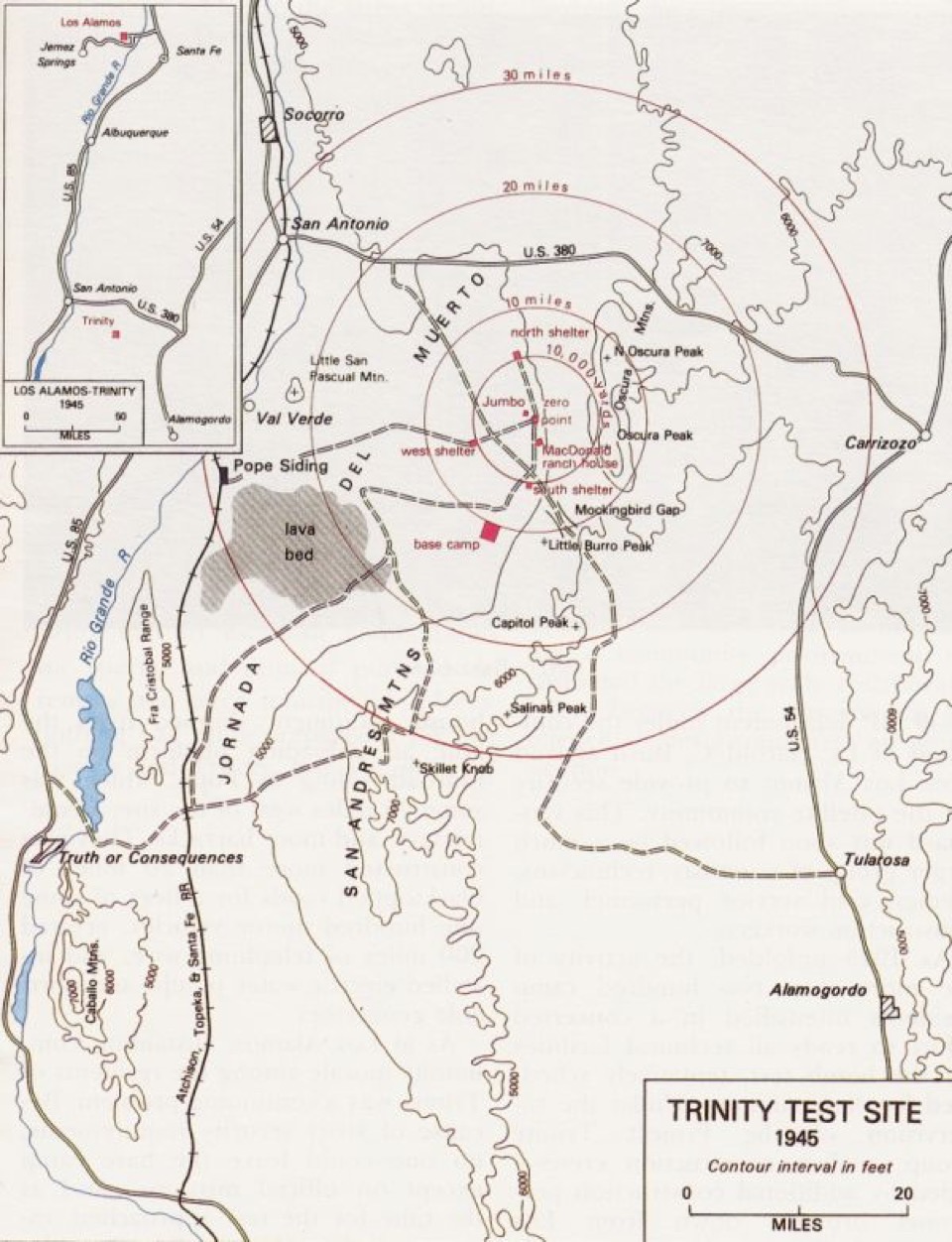
In many ways the climax of the Manhattan Project was Project Trinity, the crucial test, the first atomic bomb.
Some of the scientific staff members, including Captain Parsons, strongly favoured the gun rather than the implosion principle as more feasible for developing a usable fission weapon. They pointed out that the well-established mechanical techniques of the gun made this weapon type almost certain to work if properly designed and that the design and engineering of the outer configuration and mechanics of the gun were already well advanced.
In early 1944, the laboratory intensified procurement efforts for specialised equipment for implosion testing. By then, implosion development had made giant strides, but still unknown were the relative efficiency of such a design and how long it would take to build a moderately effective implosion device.
But the sense of having achieved substantial progress in weapon design and fabrication was marred by a number of uncertainties. The feasibility of implosion had yet to be demonstrated and the rate at which uranium-235 and plutonium-239 could be produced by the Clinton and Hanford plants remained very much in question.
Through the remaining months of 1944 and the first half of 1945, programs to perfect the uranium gun and implosion principle absorbed the major energies and resources of the reorganised laboratory. As predicted by the Los Alamos scientists, development of the gun moved ahead smoothly with few serious problems. Experiments by the laboratory's physicists proved the correctness of earlier estimates of the critical mass of the uranium-235 metal required for the gun and the gun group conducted successful firing tests, using a full sized tube and substituting uranium-238 for uranium-235.
Implosion, by way of contrast, continued to be afflicted with doubts and uncertainties. Progress toward achieving sufficient symmetry in implosion was discouragingly slow. Of the various implosion bomb designs, that proposing the use of explosive "lenses" appeared most feasible. Results of the first tests in December 1944 were so unpromising that Groves and Conant concluded that uranium-235 should not be used in an implosion bomb but be conserved for the certain-to-work gun.
As the new year opened, surprising developments dispelled the lingering air of discouragement. On February 27, 1945, the gun group finally had frozen design on the uranium-235 weapon, indicating a usable model would be ready by July 1945. Implosion also had made notable progress, and it was decided to manufacture the implosion model favoured by Oppenheimer. And to ensure at least one implosion bomb test with active material by July 4, 1945, Oppenheimer also decided to use the California Institute of Technology's Project Camel facilities for the construction of a second model with alternate design features. At this juncture, with data from Hanford indicating that shipments of plutonium in quantity would begin to arrive at Los Alamos in May 1945, with experiments on accurate establishment of the critical measurements on plutonium-239 in progress at the Metallurgical Laboratory, and with construction of a much larger plant for final purification of plutonium at Los Alamos well under way, the Trinity test date now appeared feasible.
Project Camel was the codename given to work performed by the California Institute of Technology (Caltech). These activities included the development of detonators, testing of bomb shapes, and the Salt Wells Pilot Plant (which I think is now part of China Lake), where explosive components of nuclear weapons were manufactured.
Project Trinity was the final step of the Los Alamos weapon program, the culmination of the laboratory's reorientation from research and experimentation to engineering, fabrication, and testing of an atomic device. Without Trinity, without the test of the bomb, the feasibility of employing the new weapon appeared to be much more questionable. "If we do not have accurate test data from Trinity", Oppenheimer and Kistiakowsky had warned, "the planning of the use of the ‘gadget’ over the enemy territory will have to be done substantially blindly". As 1945 unfolded, the Trinity mission became the central focus for the scientists at Los Alamos. With the bomb test now first priority, the tempo and intensity of Trinity preparations increased dramatically.
In the critical months of early 1945, making the 'gadget' work consumed the energies of both the bomb builders and Army leaders. While the scientists worked at perfecting implosion assembly and field teams prepared the remote Trinity test site at Alamogordo, General Groves and his new deputy commander, Brig. Gen. Thomas Francis Farrell, devoted much time to overseeing Trinity preparations.
The Germans Surrendered on May 7, 1945
This turned out to be the greatest crisis of conscience for the scientific community. Now, "the compelling reason for creating this weapon with such speed, our fear that Germany had the technical skill necessary to develop such a weapon, and that the German government had no moral restraints regarding its use", was gone.
The bomb was developed to stop Hitler and save the sovereign nations, and was therefore supposed to be a weapon of defence.
Work Continues in Los Alamos
As procurement crises built up in April and May 1945, Groves personally intervened in expediting requisition of lenses for the implosion bomb and globe-shaped container shells ('pumpkins') for imploding test devices.
General Farrell represented the Army at Trinity's first major event on May 7, 1945, a rehearsal shot of 100 tons of high explosives combined with a very small amount of radioactive fission materials atop a 20-foot platform.
The 100-Ton Test, involved detonating 100 tons of TNT with radioactive material added to it, so scientists could track the airborne debris, in addition to measuring the force of the blast. When the TNT was detonated on May 7, 1945, it was the largest measured blast to that point in time.
It was a successful trial run and it gave the various Project Trinity teams practical experience in performing their assignments under difficult field conditions, demonstrated a need for improvements in the transportation and communications facilities, helped calibrate instruments, and provided a likely indication of the amount of radioactive materials needed for the final test.
Although July 4, 1945, had been set as the target date for the test, few scientists at Los Alamos were convinced it could be met. Precise scheduling depended upon bringing a tremendous number of factors into proper juxtaposition, including weather, procurement of key components and equipment, production and shipment of active material, preparation of many experiments, and arrangement of security and safety measures. In mid-June 1945, Oppenheimer announced to the laboratory's group leaders that July 13, 1945, was the earliest possible date, with up to ten days later not unreasonable. He based his estimate upon information provided by the laboratory's 'Cowpuncher Committee', which had primary responsibility for coordination and scheduling of Trinity.
Following another review of developments on June 30, 1945, this committee advanced the test date to July 16, 1945, to permit inclusion of certain additional vital experiments. Two days later, Oppenheimer indicated to Groves that the laboratory leaders finally had agreed on the July 17, 1945. Groves, however, objected to the later date, pointing out that the situation in Washington required an earlier date.
With the end of the war in Europe, Secretary Stimson was scheduled to depart in early July 1945, for the Potsdam Conference, with sessions starting on the July 16, 1945. The Manhattan commander undoubtedly had conferred with Conant, Tolman, and Stimson's assistants, George Leslie Harrison and Harvey Hollister Bundy, all of whom favoured carrying out the test on the July 14, 1945. Final preparations advanced well, and Oppenheimer called Groves fixing the date for the test on the July 16, 1945.
All Systems Go!
On July 12, 1945, two scientists from Los Alamos arrived in an Army sedan with the plutonium-239 core for the implosion device. The next day a convoy came from 'the Hill' with the non-nuclear components, including the high explosives. With all components in place except the detonating system, workers lifted the device to a metal shed on a platform at the top of the tower. The detonator group then completed the firing circuit and other technicians added the instrumentation for the experiments. By five in the afternoon of the July 14, 1945, the device was ready for the test.

The next day, a Sunday, Trinity crews carried out last-minute inspections and observers checked into the Base Camp, about 10 miles south of the test tower. OSRD Director Vannevar Bush and Conant arrived from Pasadena with General Groves. Army sedans brought Charles Thomas from Santa Fe and Ernest Lawrence, Sir James Chadwick, and New York Times science reporter William L. Laurence, as well as others, from Albuquerque. Compton had decided not to come. Tolman and General Farrell were already on hand. The large contingent from Los Alamos, aboard three buses, did not reach Trinity until shortly before 03:00 of July 16, 1945, barely in time for the originally scheduled zero hour, 04:00. They stepped out into blustery and rainy weather with occasional flashes of lightning, not the clear skies and moderate winds the Trinity meteorologists had predicted.
Oppenheimer and Groves had reviewed the weather situation at midnight and then had gone forward from the Base Camp some 7,000 yards to the control dugout (10,000 yards from the test tower) to wait with Farrell, physicist Kenneth Bainbridge, who was the leader of the bomb test team, and chief meteorologist Jack M. Hubbard, who with Oppenheimer had responsibility for making the final decision on whether to carry out the test as scheduled. As 04:00 approached and the rain continued, Groves and Oppenheimer weighed the risks of going ahead, the likelihood of heavier radioactive fallout at some points, electrical failures from dampened circuits, and poor visibility for the observation airplanes. They decided to delay the shot an hour and a half. The rain stopped at four and shortly before five, with wind still blowing in the right direction, they gave the go-ahead signal for the test.
As the final countdown began, Groves left Oppenheimer and Farrell in the control dugout and returned to the Base Camp, a better point of observation and in compliance with the rule that he and Farrell must not be together in situations where there was an element of danger. At approximately the same time, the five Trinity scientists who had been guarding the test device drove away in their jeeps as bright lights illuminated the tower to foil any would-be saboteurs.
Precisely at 05:30, an automatic firing mechanism actuated the implosion device. Data from hundreds of instruments recorded what occurred in that desolate stretch of the Jornada del Muerto valley: the dawn of the atomic age. It began with a brilliant yellow light that suffused the remotest recesses of the Trinity site and was seen as far away as Albuquerque and Los Alamos to the north, Silver City (New Mexico) to the west, and El Paso (Texas) to the south. With the light came a sensation of heat that persisted even as a huge ball of fire, like a rising sun, took shape, then transformed quickly into a moving orange and red column. Out of this broad spectrum of colours rose a narrower column that rapidly spilled over to form a giant white mushroom cloud surrounded by a blue glow. Only as the glow began to fade did observers at the base camp feel the pressure of the shock wave, but its rumble reverberated for more than five minutes in the surrounding hills.
The effects of this explosion on eyewitnesses were as varied as the observers themselves. What General Farrell, for example, saw and heard from the control dugout was "unprecedented, magnificent, beautiful, stupendous and terrifying. . . . The whole country was lighted by a searing light with the intensity many times that of the midday sun. It was golden, purple, violet, grey and blue. It lighted every peak, crevasse and ridge of the nearby mountain range with a beauty . . . the great poets dream about. . . . Thirty seconds after, the explosion came . . . followed almost immediately by the strong, sustained, awesome roar which warned of doomsday. . . ."

The 'Gadget' partially uncovered, and complete, sitting on the top of the tower.
Groves permitted himself only a fleeting moment of relaxation. Less than half an hour after the test shot he called his secretary in Washington, D.C., to inform George Harrison so that he could pass on word of the test to Stimson in Potsdam. Groves reported that the strength of the explosion was at least "satisfactory plus" and perhaps far greater than estimated. Stimson, Truman, Churchill, and other Allied leaders at Potsdam were quick to realise that this preliminary evidence of the enormous power of the Trinity explosion, followed soon by more detailed substantiating data from General Groves, had introduced a new factor that would profoundly affect not only their own deliberations on how to end the war with Japan but also the whole course of international relations in the postwar world.
What a Successful Test Meant

The explosion of an implosion device on July 16, 1945, at Trinity provided final confirmation to America's wartime leaders that employment of an atomic weapon in the war with Japan was indeed a strategic reality. Until 1945, the Army's super secret atomic weapon program had not been a factor in strategic planning for carrying on the war, either in Europe or in the Pacific. The successful Allied operations against Germany in the summer of 1944 portended that country's imminent collapse and obviated the need for an atomic weapon to end the conflict in Europe. Because of these developments, Manhattan Project leaders thus considered using the bomb in the war in the Pacific and accelerated preliminary planning with the U.S. Army Air Forces (an independent U.S. Air Force was only created in 1947) for a possible atomic bombing mission against Japan.
Preparations for the tactical employment of an atomic weapon against Japan had already began in late March 1944, when General Groves first met with General Henry Harley Arnold, the AAF commanding general. The Manhattan commander briefed Arnold on the current status of bomb development, and estimating the probable time when bombs would be ready for use in combat. The next question was what type of airplane would be required to transport atomic bombs. On the basis of investigations carried out at Los Alamos and Muroc Army Air Field, had concluded that a modified B-29 probably had the requisite weight-carrying capacity and range. Should the B-29, which had gone into production in September 1943, prove not feasible, Groves suggested the British Lancaster would have to be considered. This displeased Arnold, who stated emphatically that an American-made airplane should carry the bombs, and he promised to make a special effort to have a B-29 available for that purpose.
So the AAF would organise and train the requisite tactical bomb unit, which, for reasons of security, was to be as self-sustaining as possible and exercise full control over delivery of bombs on the targets selected. Manhattan would receive from the AAF whatever assistance it needed in ballistic testing of bombs and air transportation of materials and equipment. Pending completion of the fission bombs, Groves assured Wilson that, for testing purposes, Manhattan would supply the AAF with several hundred high-explosive bombs having ballistic characteristics similar to the implosion-type model.
The AAF committed itself to supply the personnel and equipment for a heavy bomb squadron, with attached special units as required, and to make available an air base in the southwestern United States for its training. In addition, it agreed to modify and complete delivery of fourteen B-29's to the squadron by January 1, 1945; to continue flight testing of implosion-type bombs, with related training under direction of Manhattan and AAF specialists; and to assist Manhattan personnel in testing equipment and assembling ballistic data.
To command the bomb combat unit, subsequently designated the 509th Composite Group and formally activated on December 17 1944, General Arnold selected Colonel Paul Warfield Tibbets, Jr. Tibbets had an outstanding record in flying heavy bombers in Europe and North Africa and had gained a special knowledge of the B-29 as a test pilot. Because of the great importance and secrecy of the 509th's mission, Arnold gave the 509th commander virtual carte blanche to select the best-qualified personnel available.
In September 1944, Colonel Tibbets began to assemble the elements of the 509th at Wendover Field, an isolated air base in western Utah with adequate security and facilities and well located for air travel to Los Alamos and the Salton Sea Naval Air Station. Tibbets formed the various elements of the 509th with the objective of making it as self-sufficient as possible. Thus, he included in the group not only a normal B-29 unit, the 393d Bombardment Squadron (VH), but also a number of supporting elements, including the 390th Air Service Group (consisting of the 603d Air Engineering and 1027th Materiel Squadrons), the 320th Troop Carrier Squadron, and the 1395th Military Police Company (Aviation). Subsequently, for special technical requirements, the 509th acquired the 1st Ordnance Squadron, Special (Aviation), and the 1st Technical Detachment, War Department Miscellaneous Group, a catchall unit comprised of both civilian and military scientists and technicians, many from the Manhattan Project but including Army, Navy, and AAF personnel.
At the beginning of September 1944, with the external shape and aircraft requirements of the three basic bomb models, one of the uranium-235 gun type (now designated “Little Boy” instead of “Thin Man”) and two of the plutonium-239 implosion type (“Fat Man”), now frozen, the AAF started training the bomb drop squadron and, with assistance from Los Alamos technicians, completed necessary modifications on the B-29. While awaiting delivery of the first planes the squadron underwent training that emphasized ground and air techniques for handling atomic bombs.
In October 1944, only days past the scheduled delivery date, the 393d received the first modified B-29's out of a production lot of fifteen (one more than originally requested). Without delay, a continuing series of essential test drops commenced at Wendover. Over the next few months, these tests furnished critical information on ballistics, electrical fusing, flight performance of electrical detonators, operation of aircraft release mechanisms, vibration, and temperatures, as well as provided bomb assembly experience. But, perhaps more importantly, they revealed certain weaknesses in the original modifications and defective performance in the flying capabilities of the big bombers.
Because B-29's were in very short supply, the AAF's lower echelons displayed some reluctance to satisfy the Manhattan request for replacement of the inadequate planes. General Arnold said that the 509th Composite Group would get as many new planes as it required. Finally, in the spring of 1945, the second lot of fifteen greatly improved versions of the B-29 reached the air base, and training and ballistic tests proceeded at a more intensive pace.
Project leaders turned their attention to establishing a base of operations for the 509th in the Pacific Theatre. The AAF recommended that leaders of the Twentieth Air Force in the Marianas, at the time the only feasible location for the 509th base, be informed of the atomic bomb mission. It was also necessary to inform the Navy commanders in the Pacific of the atomic bomb mission, as Navy support in the immediate area of operations would be indispensable. Furthermore, Admiral Chester William Nimitz, Commander in Chief, Pacific Ocean Areas (CINCPOA), had learned of the imminent arrival of the 509th in his theatre and was asking questions concerning its mission.
The airfield and port facilities under construction on Tinian were more than adequate for the atomic bomb mission and would be ready for use by the time the 509th arrived in June 1945. Furthermore, although the Army had jurisdiction over Tinian, the Navy's 6th Naval Construction Brigade was available there to build the special installations that would be needed by the mission. By end of the month, U.S. Navy Seabees were at work on the base facilities.
By mid-July, 1945, all elements of the group had reached Tinian, including the 1st Technical Detachment comprised chiefly of civilian specialists from Los Alamos, some of whom had been brought temporarily into military service. Commanded by Parsons, the detachment furnished and tested weapon components for the 509th, supervised assembly of bombs, and checked out completed units, carefully inspecting them in bomb bays before planes took off.
The 509th's combat crews continued intensive flight training. This involved practicing navigation missions to Iwo Jima and making bomb runs to nearby islands still in enemy hands, using high-explosive projectiles with the “pumpkin” shape. At the end of training, which lasted three weeks, the crews in late July 1945, began a series of combat strikes over Japan to gain familiarity with target areas and mission tactics and also to accustom the Japanese to the appearance of small formations of B-29's flying at a great altitude. Using the pumpkin-shaped bombs, the 509th achieved excellent results against enemy towns, most of which had been hit by previous B-29 strikes. These towns, Koriyama, Nagaoka, Toyama, Kobe, Yokkaichi, Ube, Wakayama, Maizuru, Fukushima, and Niihama, were in the general vicinity of those communities selected earlier as targets for atomic bombing.
The Bombing Targets
In the late spring and early summer of 1945, Manhattan and AAF representatives met in Washington and Los Alamos for the purpose of choosing targets for the 509th's atomic bombing mission. Groves, after conferring with General Arnold, and with General Lauris Norstad selected a target committee. The committee included two members of Groves's staff (General Farrell, who served as de facto chairman when Groves was not present, and Major John A. Derry), an AAF officer (Col. William P. Fisher), and five technical experts (John von Neumann, Robert R. Wilson, and William G. Penney, a member of the British team at Los Alamos, all from the Manhattan Project, and Joyce Clennam Stearns and David Mathias Dennison from the AAF.
Three meetings were held. The committee carefully considered various criteria: the maximum range for the loaded B-29 aircraft; the need for visual bombing; likely weather conditions; and expected damage. The last criterion weighed heavily on the committee, for it pointed to the necessity to select targets where the bomb would produce the maximum damage and hence have the profoundest impact upon enemy morale. Project scientists had indicated that the bomb would most likely achieve the desired results if it were dropped on densely built-up areas of significant value to the Japanese war effort. They also had emphasised that the targets should not have been bombed previously, so the effects might be assessed more accurately. The choices were Kokura Arsenal, one of Japan's largest munitions plants, covering an area of 8 million square feet; Hiroshima, a major military embarkation port and convoy assembly point with a local army headquarters, railway yards, storage depots, and some heavy industrial plants; Niigata, an important seaport with significant industrial and commercial facilities, including an aluminium reduction plant, a large ironworks, an oil refinery, and a tanker terminal; and Kyoto, with a concentrated 3-square-mile industrial area and a population of about one million people. Stimson expressed strong objection to Kyoto, noting that the city had been the ancient capital of Japan and was a place of great religious and cultural significance to the Japanese.
When the atomic bomb directive was issued to the United States Army Strategic Air Forces (USASTAF) on July 25, 1945 Nagasaki had replaced Kyoto on the target list.
The decision to use the bomb
Meanwhile, the question of military employment of the bomb against Japan came up for consideration by the Interim Committee, a temporary body appointed by Stimson in May 1945 at the urging of project leaders and with the approval of the President.
Membership was comprised of the Secretary of War (chairman); George Harrison, as alternate chairman; former War Mobilisation Director James F. Byrnes, representing the President; Vannevar Bush; James B. Conant; MIT President Karl T. Compton; Assistant Secretary of State for Economic Affairs William Lockhart Clayton; and Under Secretary of the Navy Ralph Austin Bard. The Interim Committee established a scientific panel, comprised of Oppenheimer, Fermi, Arthur Compton, and Lawrence. While recognising that use of the bomb was essentially a military matter, the panel members nevertheless offered their opinions concerning the way it should be employed and the likely effects it would have on the targets selected.
Taking a moment to reflect on the discussion of targets and effects, Secretary Stimson proffered the conclusion that the atomic bomb should be used against Japan with no advance warning and, while not restricting the target to a civilian area, should be employed in such a way as "to make a profound psychological impression on as many of the inhabitants as possible". Conant suggested that the "most desirable target would be a vital war plant employing a large number of workers and closely surrounded by workers' houses".
Among the various reports Compton received in the following two weeks was one prepared by a group of scientists under the leadership of James Franck, an outstanding German-refugee physicist who had come to the Metallurgical Laboratory from the staff of the University of Chicago. Centring on the political and social ramifications of an atomic bombing, the Franck report favoured eventual international control of atomic energy as the only safe solution. Using the bomb against Japan without adequate warning, the report cautioned, would arouse great animosity against the United States and isolate her morally among the nations of the world, making establishment of international controls much more difficult. As an alternative, the report advocated a demonstration of the bomb in an uninhabited area, pointing out that this action would not prevent later military use of the bomb against Japan, if this were necessary.
On July 21, 1945, Stimson received not only Groves's detailed report on the successful test at Trinity, but also cables from Harrison indicating that atomic bombs would be ready sooner than expected. He promptly passed the word to American and British leaders at the Potsdam Conference, including President Truman, Prime Minister Churchill, Secretary of State Byrnes (as of 3 July), General Marshall, and Lord Cherwell, all of whom were elated by the news. On July 24, 1945, Stimson showed the President the tentative plan of operations, which called for the first atomic bombing mission any time after August 1, 1945, subject to completion of preparations and suitable weather. Truman accepted the plan without reservation, for, Stimson recalled, "that was just what he wanted. . . ."
On July 25, 1945, it was decided to proceed with the atomic bombing of Japan, and the procedure was that the 509th Composite Group, 20th Air Force was to deliver its first special bomb as soon as weather permitted visual bombing after about August 3, 1945 on one of the targets: Hiroshima, Kokura, Niigata or Nagasaki. And additional bombs were to be delivered on the above targets as soon as made ready by the project staff.
On July 26, 1945 Truman and other Allied leaders issued the Potsdam Declaration outlining terms of surrender for Japan. It was presented as an ultimatum and stated that without a surrender, the Allies would attack Japan, resulting in "the inevitable and complete destruction of the Japanese armed forces and just as inevitably the utter devastation of the Japanese homeland". The atomic bomb was not mentioned in the communication. On July 28, 1945, Japanese papers reported that the declaration had been rejected by the Japanese government. The statement was taken by both Japanese and foreign papers as a clear rejection of the declaration. On August 6 and 9, 1945 the atomic bombs were released on Hiroshima and Nagasaki causing catastrophic wide spread devastation of Japan. On August 15, 1945, Japan surrendered to the Allied leaders.
Survey of the Bombing Rffects
The swift surrender of Japan opened the way for American scientific teams to survey, on the ground, the specific effects of the atomic bombing of Hiroshima and Nagasaki. Not only were scientists, medical personnel, and professional military men greatly interested in learning the results of the first employment of atomic weapons in warfare, but also the commanders of the occupation troops that were scheduled shortly to move into the two bombed cities desired a check on the possible hazards with which they might have to cope.
Negotiations with the Japanese to arrange for an early entry into Hiroshima and Nagasaki culminated in the formation of a special party, comprising mostly of medical personnel from the International Red Cross, the Army Medical Corps, MacArthur's staff, and the Manhattan Project. The special party, accompanied by two representatives of the Japanese government, flew into Hiroshima on September 8, 1945. Using Geiger counters and other instruments, members of the party checked through the destroyed area of the city, determining that no significant amounts of radioactivity persisted.
Within a radius of 1 mile of the epicentre of the explosion, destruction in both cities was virtually complete, except for the frames of a few reinforced concrete buildings.

A primary objective of the Manhattan survey teams was to ascertain the particular kinds of injuries suffered, with special attention to the effects of radioactivity. By far the largest number of casualties resulted from burns traceable to the heat of the explosion and the fires. Other major sources of injury were falling debris, the blast pressure, and radiation.
Most radiation injuries occurred from exposure of the victims to gamma rays at the time of the explosion. Early reports concluded that there was little evidence of casualties from alpha and beta rays and from residual radioactivity in the bombed-out areas.
It was also concluded that, while the bombs had some impact on the leaders of the Japanese government, their knowledge of the awesome character of the new weapon seemed not to have played a significant part in convincing them of the need to surrender.
Yet atomic bombs are still considered to have played a crucial role in ending the war. But as Gerald James Holton (professor of the history of science at Harvard) once commented, "What really won the war was radar. It was radar and the proximity fuse and above all, because it is so forgotten, synthetic rubber, without which the war could not be pursued. As a result of these successes in essentially technologies the scientists and engineers had a completely different status". One also could add sonar, aerodynamics, the internal combustion engine and communications technology to that list.
What is certain is that the Manhattan Project put “Big Science” on the map, and the atomic bomb put the physicist on top of the pile.